Antimicrobial activity of Ferula gummosa and Artemisia sieber essential oils in gaseous phase Marziye Saeediye1, Ahmad Farhad Talebi1*, Mohammad Reza Morshedloo2
1Department of Biotechnology, Faculty of New Sciences and Technologies, Semnan University, Semnan, Iran
2Department of Horticultural Science, Faculty of Agriculture, University of Maragheh, Maragheh, Iran
*Correspondence to: Ahmad Farhad Talebi
Citation: Saeediye M, Talebi AF, Morshedloo MR (2022) Antimicrobial activity of Ferula gummosa and Artemisia sieber essential oils in gaseous phase. Sci Academique 3(1): 26-34
Received: 14 May, 2022; Accepted: 21 June 2022; Publication: 25 June 2022
Abstract
Bacterial infections treatment and eradication are mainly based on exploration of new chemical antibiotics. Several clinical side effect and most importantly, emerging resistant bacteria is leading to an urgent need for uncovering alternative or complementary treatments against bacterial infections. Herbal essential oils (HEOs) extracted from Ferula gummosa and Artemisia sieberi have presented promising results for their significant inhibitory potential. The aim of this study was to compare the antibacterial activity of essential oil both in liquid and in vapor phases, against a panel of bacteria such as Staphylococcus aureus, Bacillus cereus, Escherichia coli, Shigella dysenteriae, Klebsiella pneumonia, Salmonella typhi and Pseudomonas aeruginosa. The inhibitory potential of HEOs were determined with in-vitro disc volatilization assay (DVA)in the non-contact technique and Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) with direct contact broth dilution assays (BDA). The results showed that S. dysenteriae, E. coli, B. cereus and S. typhi were similarly sensitive to F. gummosa HEO. On the other hand, E. coli, S. aureus and B. cereus showed an elevated sensitivity to A. sieberi HEO. Moreover, a significant enhancement in the bactericidal action was observed by using F. gummosa HEO as compared to those extracted from A. sieberi in DVA. In general, it has been observed that gram-positive bacteria were more sensitive than gram-negative strains against A. sieberi HEO. Evaluated microorganisms exhibited different sensitivity to tested HEOs in gaseous phase, but overall bactericidal efficiency was elevated by HEOs implementation in the vapor phase.
Keywords: Antimicrobial activity; Essential oils; Vapour phase; Non-contact assay; Inhibitory potential
Introduction
Natural resources originated from local herbs have become increasingly popular during the recent decades. The idea of exploiting secondary metabolites of plants and screening their medical potential is belonged to the traditional medicine [1]. Global antibiotic resistance and newly emerged infections has resulted in constantly-increasing insatiable global appetite for the development and introduction of herbal antibacterial compounds. These biomolecules could also be used for designing new drugs [2,3]. Moreover, the Federal Drug and Food Administration (FDA) considered the use of herbal essential oils (HEOs) as General Recognized as Safe (GRAS) and further studies demonstrate the cytotoxicity of the HEOs are scarce [4].
Initial screening of these biomolecules is substantial subject in order to reduce the global concerns about multi-drug resistant bacteria. Various in vitro inhibitory assay of herbal metabolites has been broadly investigated and demonstrated against different bacteria by direct contact; Different procedures such as gel diffusion as well as dilution techniques are successfully investigated on gram negative and gram-positive bacterial species [5-7]. However, due to hydrophobic and volatility characteristics of the HEOs and other aromatic secondary metabolites, the direct-contact assays face many problems; the challenges namely low water solubility, affinity to the media, micelles formation and consequently loss their potency in order to attachment to microorganisms [8] limit the application of the herbal antibacterial substances [9]. To overcome the diminished activity of the HEOs in aqueous media, addition of detergents, emulsifiers or solvents such as Tween 80, DMSO and methanol might alter the activity of the HEOs [9]. Moreover, direct supplementation of HEOs and their preservatives as food additives causes a sensory impact resulting to altering the natural odor and taste [10]. Hence, alongside to the classical screening methods to determine the antibacterial activity of natural compounds, another complementary methods should also be used [11]. It is worth noting that volatile nature of secondary compounds in a vapour phase (VP) had more inhibitory effect in comparison with direct contact methods [12,13].
The results of the previously published studies [14,15] confirmed that HEOs extracted from different medicinal herbs inhibited more efficiency against pathogens in the VP. This higher inhibitory effect was achieved at relatively lower concentrations comparing to the direct contact, therefore the challenges of sensory alterations can be reduced [8,16]. Non-contact antibacterial potential of HEOs not only has broad application in different fields [17], but also could be one of the fastest screening method for large quantities of HEOs with higher throughput for different bacteria [9]. Taking into consideration all above mentioned facts, this study focuses on HEOs obtained from two local medicinal herbs, i.e. Artemisia sciberi and Ferula gummosa. The inhibitory potential on a set of bacterial species was investigated during the direct contact as well as VP.
Materials and methods
Plant material
The oleo-gum-resin of Ferula gummosa, and the whole plant of Artemisia sieberi were collected from the 2 years old plants growing in Mehdishahr, Semnan, province, Iran (N 35° 42´, E 53° 21´, 1630 m a.s.l.) in June 2019, during the period of plant harvesting. In the case of A. sieberi, the leaves were air-dried in the shade at room temperature, powdered with electric mills, protected from the direct light. The oleo-gum-resin of F. gummosa was harvested from natural scraps were made on the healthy shoots and exudates were transferred to the laboratory by stainless steel containers. The collected oleo-gum-resin was dried and crushed into a soft powder and stored in refrigerator (6oC) in a dark container.
Preparation of HEOs
Air dried leafage of A. sieberi were powdered and subjected to hydro distillation for 4 h using a Clevenger-type apparatus (Ashke shishe, Iran) as previously reported [18]; 173 g of the dried weight biomass were placed in a flask (1L) and 600 ml distilled water were also added. The mixture was boiled for 4 h, then the oil was decanted, dehydrated with anhydrous sodium sulphate and collected in amber vials that were kept at −4 °C till their biological activity assay. The oil yield was estimate on dry matter (v/w). The oleo-gum-resin of F. gummosa (15g) was inserted in a bag with large pores, then placed in a flask (1L) with 700 ml distilled water. The process of hydro distillation was performed as described above for 3 h.
Microorganisms and growth conditions
The tests were performed against 7 bacterial strains. Gram-positive bacteria were Staphylococcus aureus ATCC 25923, and Bacillus cereus PTCC1015. Gram-negative bacteria were Escherichia coli ATCC25922, Shigella dysenteriae ATCC12678, Klebsiella pneumonia ATCC13883, Salmonella typhi PTCC1609 and Pseudomonas aeruginosa ATCC27853. All bacterial strains were grown in Mueller-Hinton (MH) agar and Nutrient broth and Nutrient agar (Merck, Darmstadt, Germany). Stock cultures of bacterial strains were grown in Nutrient broth at 37 °C, 160 rpm for 18 h. The culture were subjected to adjust the turbidity of the inoculum concentration using the 0.5 McFarland standard (1.5×108 CFU/ml ) solution. The cells were suspended in sterile Nutrient broth to reach 5×105 CFU/ml for the antimicrobial tests.
Antimicrobial assay
The major task of the present study was to evaluate the antibacterial activity of the extracted HEOs in VP; so disk volatilization method was conducted and the results compared by direct-contact observations in liquid phase.
Non-contact antimicrobial assay by disc volatilization assay
In vitro antimicrobial activities were determined by non-contact method based on the method previously published [16]. The tests were performed in 60 mm petri dish (PD). MH agar was used as growth media and inoculated by spreading 50 μL of the prepared suspensions containing approximately 5×105 CFU/ml of different bacterial strains. Sterilized paper discs (6.4 mm in diameter ) were soaked in 350 μL pure HEOs of A. sieberi and F. gummosa for 30 min and placed in the inside surface of the lid of the PD. Finally, the inoculated PD was closed with its lid containing impregnated disc and hermetically sealed with parafilm to prevent leakage of HEO vapor. Positive control was left by untreated discs. The Petri dishes were incubated at 37 °C and the bacterial cells were exposed to the HEOs vapors for 18 h. After incubation, survival count was determined and the results were compared by its control. All tests were carried out in triplicate.
Determination of minimum inhibitory concentration (MIC)
The direct-contact broth dilution assays were performed based on the recommendations of the Manual of Clinical Microbiology associated with modifications published before [19]. Twofold serial dilutions of HEOs were prepared from 40 to 0.078% in sterile Nutrient broth. The medium was divided within test tubes which were further inoculated with 20 μl of an overnight bacterial culture (~5×108 CFU/ml). All the tubes were incubated in triplicate for each concentration. The turbidity of the tubes (bacterial growth) was observed after 18 h incubation at 37 °C to determine minimum inhibitory concentrations (MIC). Then the tubes without turbidity plated out on nutrient agar and incubated for 24 h. The number of bacterial colonies was compared with the controls and then the values of the minimum bactericidal concentrations (MBC) were also determined.
The MBC is the lowest concentration of the HEOs required to kill the cells. The MIC values were determined as the minimal concentration of HEOs that could completely inhibit visible growth of bacterial strains in comparison with the control after exposure to HEOs. The control tube of the bacterial growth contained no HEOs. Nutrient broth containing mentioned HEO was observed as pollution control.
Results
Antimicrobial assay
HEOs extracted from A. sieberi and F. gummosa were evaluated for their antimicrobial potential by Broth macro dilution and non-contact methods against seven bacterial strains.
Broth macro dilution assay (BDA)
In this method antimicrobial potential of HEOs was studied in liquid phase. MIC and MBC values of HEOs were summarized in Tables 1. MIC values of the studied HEOs are expressed in percent. Both of A. sieberi and F. gummosa HEOs presented inhibition potent against all of the bacterial strains. A. sieberi had morepotent inhibition activity than F. gummosa.. The most sensitive strain to A. sieberi was S. aureus (MIC: 5%) while F. gummosa produced the highest MIC value against this pathogen (MIC: 40%). In the case of P. aeruginosa, equalMIC values were observed for Both of the HEOs (40%). The results showed that S. dysenteriae, E. coli, B. cereus and S. typhi were similarly sensitive to F. gummosa HEO. On the other hand, E. coli, S. aureus and B. cereus showed an elevated sensitivity to A. sieberi HEO. Moreover, the results depicted that, HEO extracted from F. gummosa had equal MIC and MBC values for all tested bacteria. In general, it has been observed that gram positive bacteria were more sensitive than gram negative strains against A. sieberi HEO.
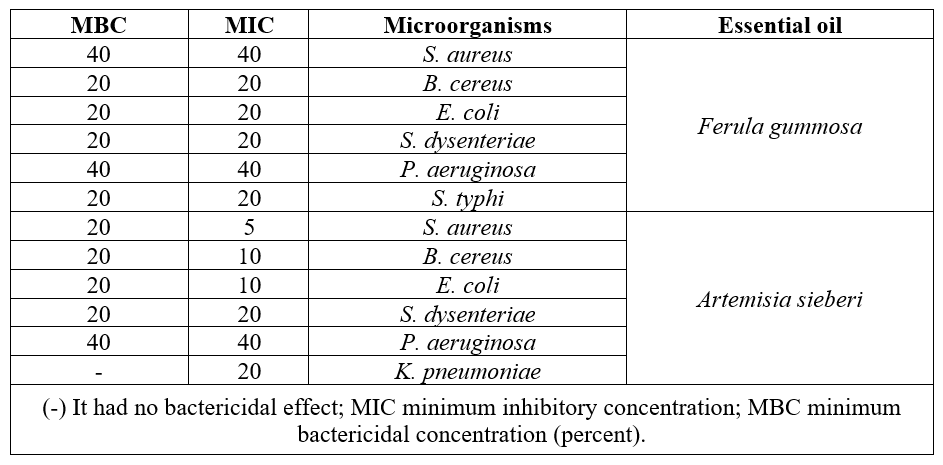
Table 1: Antibacterial activity of HEOs against some bacterial strains by broth macro dilution.
Disc volatilization assay (DVA)
Comparative investigations of antibacterial potential of the HEOs were also conducted in the VP (Table 2). HEO obtained from A. sieberi had inhibitory potential with reduce in colony number against studied gram positive bacteria. 0n the contrary, this HEOs had no inhibitory effect against gram negative strains such as E. coli in VP.
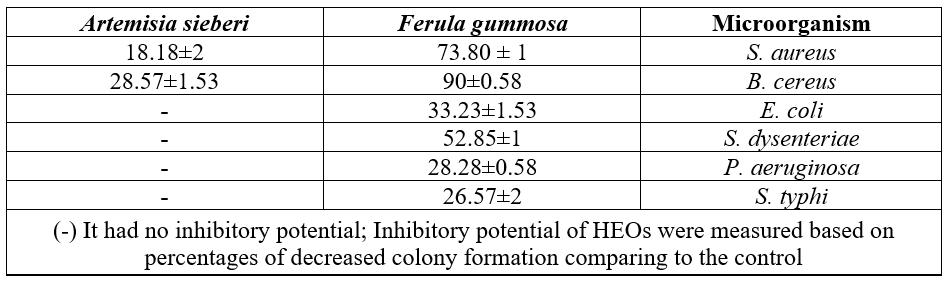
Table 2: Antibacterial activity of studied HEOs against gram positive/negative bacterial strains by Disc volatilization assay.
Inhibitory potential of F. gummosa HEO was considerable. , The most antibacterial effects were observed against gram positive bacterial species; Colony number of S. aureus and B. cereus reduced by 73.80% and 90% in comparison with the control, respectively. Antibacterial activity of Ferula HEOs was also considerable against gram negative bacteria such as E. coli, S. dysenteriae, S. typhi and P. aeruginosa compared to the control by 33.32%, 52.85%, 26.57% and28.28%, reduction, respectively. S. typhi was in general less susceptible to Ferula HEOs compared to B. cereus as only in one case without MIC records.
Discussion
In this study indirect antimicrobial evaluation was performed to combat against the high hydrophobicity and irreversible changes in HEOs in aqueous culture medium,. The physicochemical properties of extracted aroma antibiotics face with sudden changes in response to differences in polarity of the solvents [7]. This phenomenon might also alter the inhibitory potential of aroma antibiotic substances [9,20]. Therefore, an effective technique is applied herein, to overcome the shortcomings caused by the contact method. Disc volatilization of HEOs in VP, potentially enhance the inhibitory potential of aromatic and antibiotic properties of HEOs extracted from F. gummosa and A. sciebri. Therefor both of herbal aroma antibiotic and development of non-contact assay could be promising approaches to support the treatment of bacterial infections [11].
The inhibitory potential of HEOs in liquid phase was also determined with direct contact method; however, the inhibitory effect of HEOs determined with non-contact disc volatilization assay were significantly higher. According to the result of the non-contact and direct contact antibacterial assays, A. sciebri and F. gummosa HEOs showed inhibitory potential in both vapor and liquid phase. According to our results in liquid phase, A. sciebri HEO had inhibitory potential against both Gram-positive and Gram negative bacteria, which was parallel to previous publication [21]. In liquid system, A sciebri HEOs was the most effective against gram positive bacteria, which was in accordance with previously published results [22]. In the liquid phase, F. gummosa HEO, showed similar inhibitory potential for all tested bacteria, in contrast with our observations, [23] reported that, gram positive bacteria were more sensitive than the gram-negative bacteria. MIC of F. gummosa HEOs was equivalent to MBC for the all tested bacteria, which indicates the bactericidal property [23]. Although, differences probably caused by the compositions of the oleo gum resin oils and the type of species might impose some variation reported in different experiments [11,24].
In the VP, Gram-positive bacteria were more sensitive to the herbal aroma antibiotic treatment compared to Gram-negative bacteria. Our observations were in accordance with previous reports [25], Minor difference is probably caused by the compositions of the herbal secondary metabolite and tested bacteria in the experiments [26,11]. Gram-negative bacteria are generally less susceptible to EOs than Gram-positive bacteria because the outer membrane of Gram-negative bacteria contains hydrophilic lipopolysaccharides that create a barrier against macromolecules and hydrophobic compounds, providing a higher tolerance of hydrophobic antimicrobial compounds such as those present in EOs [27].
Herbal essential oils possess various number of chemical metabolites especially monoterpenes, which inhibit the growth and proliferation of wide variety of pathogenic microorganisms. HEOs have high level of phenolic compounds, aldehydes, and alcohols, such as carvacrol, eugenol, and thymol, which show significant antimicrobial potential against pathogens [28]. Carvacrol is one of the constituents of Artemisia EO, increase membrane fluidity and cause the leakage of protons and potassium ions, resulting in a collapse of membrane potential and inhibition of ATP synthesis [12].
Hydrophobic character of HEOs is due to its potential to change in the cell wall lipid structure and inner membrane, disturbing cellular structures leading to protein denaturation and increasing cell membrane permeability, which is associated with ions leak out and decrease of membrane potential, collapsing the proton pump and depleting the ATP pool and eventually microbial death [16]. Cell membrane disruption followed by reduction in PH , and consequently control of DNA transcription, protein synthesis and enzyme activity would lost [12]. Some of HEOs or their components possess an anti-plasmid effect; that means prevent from emergence of antibiotic resistant strains since extra-chromosomal DNA sequences cannot be shared among pathogens through the plasmid [16].
Wild aromatic medicinal plants specially F. gummosa and A. sieberi are introduced as renewable and naturalmajor source of new antibacterial compounds. In many researches, which have been shown that the α-pinene, β-pinene, menthol, myrthenole, and benzene are most anti pathogenic fractions of F. gummosa and A. sieberi, which were evaluated. Oily constituents of F. gummosa, thujone, terpinolene, camphor, α-humulene, camphene, β-caryophyllene, cadinene, verbenone, 1,8-cineole and camphor have good antibacterial activity against Gram-positive and Gram-negative bacteria [4]. So many investigations have mentioned that the antibacterial potential of F. gummosa can be depended to their terpenoids and flavonoids in EO extracted [29,30]P. aeruginosa is an opportunistic pathogen of immunocompromised hosts by high resistance to antimicrobials compounds has successfully inhibited by F. gummosa HEO. Although numerous investigation were unable to control this pathogen in direct-contact assay by F. gummosa HEO [31,32,33]. S. dysenteriae infections are one of the most contagious bacterial diarrhea diseases that can cause widespread epidemics with high mortality [34]. According to the result of the present study, ferula oleo-gum-resin against this bacterium showed the high inhibitory potential to 52.85%.
In the case of E. coli, Ferula HEO could significantly decrease the growth by one-third, contrary to our result, kavoosi et al. reported that F. assafoetida EOs in the contact assay had no inhibitory effect against E. coli [35]. The difference is probably caused by different method of antibacterial assay and differences in preparation of secondary metabolites and their composition.
S. aureus is a leading cause of health care-associated infections world- wide. Despite active surveillance efforts, advances in the prevention of infection and new antibiotics, methicillin-resistant S. aureus (MRSA) remains a prominent pathogen associated with high rates of mortality [36]. At the present study it should be highlighted that aromatic phase of F. gummosa HEO showed significant inhibitory effect till 73.80% against this bacteria. According to the obtained results, the antimicrobial activity of this aroma antibiotic compound can be attributed to monoterpene hydrocarbons [23]. Also in the case of B. cereus, F. gummosa HEO had considerable potential to 90% infection inhibition.
In conclusion, we must highlight HEO extracted from F. gummosa as the most active secondary metabolite in the VP in comparison with A. sieberi pure HEO that had moderate activity just against gram positive bacteria. Because of the various bio-compounds presented in HEOs, it seems they have no specific cellular targets [31]. The antimicrobial action of HEOs is determined with their hydrophilic or hydrophobic nature, chemical components, type of microorganism and eventually the method of implementation [32].
Conclusions
The inhibitory efficacy of herbal secondary metabolites against various bacteria by direct-contact antibacterial assay has been extensively demonstrated. However, investigation of HEOs in VP has not been thoroughly understood using newly emerged indirect contact techniques. Hence, based on our results, we suggest that non-contact antibacterial assay provides the best detection for the both properties; volatility and inhibitory potential of aromatic oily liquids based on vapor phase.
Inhibitory potential of HEOs were successfully examined. In conclusion , F. gummosa HEO showed dramatic antibacterial efficacy in VP against all tested bacteria and the EOs extracted from this plant could be considered as an alternative or enhancer to the present antibiotics in various industries [37]. in the contrary, A.sciberi HEO was more potent inhibitor in liquid phase.. The results showed that HEOs have different properties in liquid or vapor phases which results in diverse biological activity [11]. Comparison between BDA and DVA results confirms that non-contact antibacterial assay could be a promising alternative or complementary method to eliminate the shortcomings caused by contact methods.
Acknowledgements: The authors would like to thank Semnan University for funding this study.
References
- Pavela R, Morshedloo MR, Lupidi G, Carolla G, Barboni L, et al. (2020) The volatile oils from the oleo-gum-resins of Ferula assa-foetida and Ferula gummosa: A comprehensive investigation of their insecticidal activity and eco-toxicological effects. Food Chem Toxicol 140:111312.
- Gandhi GR, Leão GC de S, Calisto VK da S, Vasconcelos ABS, et al. (2020) Modulation of interleukin expression by medicinal plants and their secondary metabolites: A systematic review on anti-asthmatic and immunopharmacological mechanisms. Phytomedicine 70: 153229.
- Tran N, Pham B, Le L (2020) Bioactive Compounds in Anti-Diabetic Plants : Biology (Basel) 9: 1–31.
- Reyes-Jurado F, Cervantes-Rincón T, Bach H, López-Malo A, Palou E (2019) Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind Crops Prod 131: 90–95.
- Hashemi Karuie SM, Salehi M, Asghar Heidari M, Mobiny M, Nasrollahi Omran A (2016) Effect of Aqueous and Alcoholic Extracts of Roots of Ferula Gummosa Boiss on Growth of Some Bacterial Pathogens. Journal of Neyshabur University of Medical Sciences 4: 10-18.
- Satarian F, Hosseini HM, Ghadaksaz A, Amin M, Fooladi AAI (2018) Multi-Drug Resistant Clinical Pseudomonas aeruginosas Inhibited by Ferula gummosa Boiss. Recent Pat Antiinfect Drug Discov 13: 89–99.
- Salehi M, Hashemi Karuie SM, Nasrollahi Omran A, Mobiny M, Asghar Heidari M (2015) Antifungal activity of in vitro aqueous and alcoholic extracts of Barije root (Ferula gummosa)]. Journal of Birjand University of Medical Sciences 21: 444-450.
- Boukhatem MN, Ferhat MA, Kameli A, Saidi F, Mekarnia M (2014) Liquid and vapour phase antibacterial activity of Eucaptus Glibulus essential oil Suscebtibility of selected respiratory tract pathogens. AJID 10: 105-117.
- Kloucek P, Smid J, Frankova A, Kokoska L, Valterova I, et al. (2012) Fast screening method for assessment of antimicrobial activity of essential oils in vapor phase. Food Res Int 47: 161–165.
- Velázquez-Nuñez MJ, Avila-Sosa R, Palou E, López-Malo A (2013) Antifungal activity of orange (Citrus sinensis var. Valencia) peel essential oil applied by direct addition or vapor contact. Food Control 31: 1–4.
- Ács K, Balázs VL, Kocsis B, Bencsik T, Böszörményi A, et al. (2018) Antibacterial activity evaluation of selected essential oils in liquid and vapor phase on respiratory tract pathogens. BMC Complement Altern Med 18: 1–9.
- Fisher K, Phillips C (2008) Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Technol 19: 156–164.
- Inouye S, Uchida K, Maruyama N, Yamaguchi H, Abe S (2006) A Novel Method to Estimate the Contribution of the Vapor Activity of Essential Oils in Agar Diffusion Assay. Nippon Ishinkin Gakkai Zasshi 47: 91–98.
- Inouye S, Uchida K, Abe S (2006) Vapor activity of 72 essential oils against a Trichophyton mentagrophytes. J Infect Chemother 12: 210–216.
- Tyagi AK, Malik A (2011) Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapour phase against food spoiling microorganisms. Food Control 22: 1707–1714.
- Reyes-Jurado F, Navarro-Cruz AR, Ochoa-Velasco CE, Palou E, López-Malo A, et al. (2020) Essential oils in vapor phase as alternative antimicrobials: A review. Crit Rev Food Sci Nutr 60: 1641–1650.
- Wu K, Lin Y, Chai X, Duan X, Zhao X, et al. (2019) Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci Nutr 7: 2546–2555.
- Santomauro F, Donato R, Pini G, Sacco C, Ascrizzi R, et al. (2018) Liquid and Vapor-Phase Activity of Artemisia annua Essential Oil against Pathogenic Malassezia spp. Planta Med 84: 160–167.
- Ács K, Bencsik T, Böszörményi A, Kocsis B, Horváth G (2016) Essential oils and their vapors as potential antibacterial agents against respiratory tract pathogens.
- Das S, Vörös-Horváth B, Bencsik T, Micalizzi G, Mondello L, et al. (2020) Antimicrobial activity of different artemisia essential oil formulations. Molecules 25.
- Aati HY, Perveen S, Orfali R, Al-Taweel AM, Aati S, et al. (2020) Chemical composition and antimicrobial activity of the essential oils of Artemisia absinthium, Artemisia scoparia, and Artemisia sieberi grown in Saudi Arabia. Arab J Chem 13: 8209–8217.
- Mahboubi M, Farzin N (2009) Antimicrobial activity of Artemisia sieberi essential oil from central Iran. IJm 1: 43-48.
- Abedi D, Jalali M, Asghari G, Sadeghi N (2008) Composition and antimicrobial activity of oleogumresin of Ferula gumosa Bioss. essential oil using Alamar Blue (TM). Res Pharm Sci 3: 41–45.
- Younessi-Hamzekhanlu M, Farmani B, Alirezalu K, Fathizadeh O, Sabzi nojadeh M (2019) Study of phytochemical composition and antibacterial effects of Artemisia fragrans Willd. Essential oil in different seasons]. JFST 16: 357-367.
- Doran AL, Morden WE, Dunn K, Edwards-Jones V (2008) Vapour–Phase Activities of Essential Oils against Antibioticsensitive and Resistant Bacteria Including MRSA. Lett Appl Microbiol.
- Mulyaningsih S, Sporer F, Zimmermann S, Reichling J, Wink M (2010) Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine: 1061-1066.
- Hyldgaard M, Mygind T, Meyer RL, Hayashi MAF, Knapp C (2012) Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components.
- Clemente I, Aznar M, Silva F, Nerín C (2016) Antimicrobial properties and mode of action of mustard and cinnamon essential oils and their combination against foodborne bacteria. Innov Food Sci Emerg Technol 36: 26–33.
- Lorenzi H, Matos FJ (2002) Plantas medicinais no Brasil : nativas e exóticas.
- Kotan R, Cakir A, Dadasoglu F, Aydin T, Cakmakci R, et al. (2010) Antibacterial activities of essential oils and extracts of Turkish Achillea, Satureja and Thymus species against plant pathogenic bacteria. J Sci Food Agric 90: 145–160.
- Eftekhar F, Yousefzadi M, Borhani K (2004) Antibacterial activity of the essential oil from Ferula gummosa seed. Fitoterapia 75: 758–759.
- Iranshahi M, Hassanzadeh-Khayat M, Bazzaz BSF, Sabeti Z, Enayati F (2008) High content of polysulphides in the volatile oil of ferula latisecta rech. F. et Aell. Fruits and Antimicrobial Activity of the Oil. J Essent Oil Res 20: 183–185.
- Asili J, Sahebkar A, Bazzaz BSF, Sharifi S, Iranshahi M (2009) Identification of essential oil components of ferula badrakema fruits by gc-ms and 13c-nmr methods and evaluation of its antimicrobial activity. J Essent Oil-Bearing Plants 12: 7–15.
- Ussery DW, Sekse C, Sváb D, Falgenhauer L, Horváth B, et al. (2021) Genome Analysis of a Historical Shigella dysenteriae Serotype 1 Strain Carrying a Conserved Stx Prophage Region.
- Mohammadhosseini M, Venditti A, Sarker SD, Nahar L, Akbarzadeh A (2019) The genus Ferula: Ethnobotany, phytochemistry and bioactivities – A review. Ind Crops Prod 129: 350–394.
- Lepelletier D, Maillard JY, Pozzetto B, Simon A (2020) Povidone iodine: properties, mechanisms of action, and role in infection control and Staphylococcus aureus decolonization. Antimicrob Agents Chemother 64: 1-13.
- Shahverdi AR, Fakhimi A, Zarrini G, Dehghan G, Iranshahi M (2007) Galbanic Acid from Ferula szowitsiana Enhanced the Antibacterial Activity of Penicillin G and Cephalexin against Staphylococcus aureus. Biol Pharm Bull 30: 1805–1807.


