Review: UV protection and anticancer properties of lichen secondary metabolites Mariraj Murugan1*, Shenbagam Muthu1, Kalidoss Rajendran2, Ponmurugan Ponnusamy1
1Biomedical Research Lab, Department of Botany, Bharathiar University, Coimbatore, India
2Assistant professor, Department of Biotechnology, Sri Kaliswari College, Sivakasi, Tamil Nadu, India
*Correspondence to: Mariraj Murugan
Citation: Murugan M, Muthu S, Rajendran K, Ponnusamy P (2021) Review: UV protection and anticancer properties of lichen secondary metabolites. Sci Academique 2(1): 68-96.
Received: 04 May, 2021; Accepted: 26 May 2021; Publication: 31 May 2021
Abstract
The organism consisting of algae and fungi together in one single thallus is called lichens, yet they are also the subject of study by the lichenologists because either the purified secondary metabolites or the crude extract of lichen have a wide variety of biological activities such as anticancer, antibiotic, anti-inflammatory, antioxidant, anti-fungal, anti-HIV, etc., The following review article focused primarily on scientific proof for the documented evidence of medicinal value of lichen compounds. The present article also made the review on the secondary metabolites of lichens from traditional drug research, ultraviolet radiation protection and anti-cancer treatment, and summarized the biological activities of the main functional compounds such as atranorin, calycin, pannarin, parietin and usnic acids, etc. Finally, it is concluded that these compounds have powerful sunscreen and anticytotoxic effects both in vivo and in vitro.
Keywords: Sunscreen compound; Lichen secondary metabolites; anticancer
Introduction
Lichens comprises a group of organisms which are fungus (mycobiont) in one hand and photosynthetic eukaryotic algae (photobiont) or cyanobacteria on the other [1] and sometimes actinobacteria [2]. By their water retention property, fungi nurture algae with water but their photosynthetic nature, the algae cherish fungi with food. The association is referred to as symbiosis. The fungi which occupy 90 per cent of total lichen thallus while the algal (cyanobacteria or blue green algae) layer occupies only 10 percent. About 20,000 lichen species are found to be reported all over the World. Nearly 8 percent of the land surface of earth are covered with lichens. The most distinguishing characteristic of this Lichen is its slow growth rate of a few species which is indicated by the growth of just 1 cm in 10 years. Therefore, the lichen has to play a role in selecting variety of a growth forms capable of thriving under extreme conditions such as UV radiation, high temperature, excess salt, drought environment. The nutraceutical value of lichens is also known from the previous literature [1]. Most lichen forms can produce chemical substances. These substances can exert their biological activities, regulation of the synergism between symbionts and environmental interactions [3]. The chemical substances are generally divided into primary and secondary metabolites. Presence of primary metabolites from both the symbionts necessarily means that they are essential for structural functions and cellular metabolism. Secondary metabolites are generally fungal derived [4–7] and therefore stored in different parts of lichen like upper cortex, lower cortex, medulla, sexual and asexual fruiting bodies. But, photobiont and mycobiont metabolites interaction are needed to produce secondary substances. Lichen secondary metabolite production cannot be identified in a naturally occurring non symbiotic state [8]. This has aroused further interest in the study of the bioactive compounds of lichen associated bacteria, especially Actinobacteria and Cyanobacteria [9].
Sun which emits ultraviolet radiations which are the type of electromagnetic non ionizing radiation. Both UV B and UV A are the most damaging to living things has been reported by the National Toxicology program on Carcinogens. As many as 90% of total skin cancer cases showed UV exposure due to solar radiation [10]. Skin cancer causes uncontrolled growth of skin cells. UV A and UV B radiations considerably affect UV exposed skin cells as well as induce genetic defects or mutation. It triggers biochemical changes that lead the skin cells to form malignant tumors. There are many skin cancers types. These are Basal cell carcinoma (BCC), Squamous cell carcinoma (SCC), and Melanoma. BCC and SCC are not serious type cancer treatable but melanoma cancer is a serious type of cancer treatment is crucial. There were 1,80,78,957 persons affected by skin cancer as of 2018 records and it causes considerable death which raises to about 95,55,027 patients [11]. Nowadays, most are vulnerable to skin cancer throughout the world. The ignorance of skin cancer is a serious problem and it has been identified from several cases that the affected are in great danger. Thus, documentation of skin cancer is in need. The case studies of death reports caused by Basal cell carcinoma (BCC), Squamous cell carcinoma (SCC), are very rare and It is estimated that almost 30% of affected deaths are caused by aggressive Melanoma type of skin cancer [12,13]. Lichens produce more than 1000 secondary metabolites. There are very useful biological activities such as anticancer, antibiotic, anti-inflammatory, antioxidant, anti-fungal, anti-HIV, etc., used for preventive measures in medicine are known to be attributed to lichen compounds [2, 14,15]. Besides many other applications, UV proof metabolites such as Depsidone derivatives, Depside derivatives, Xanthone derivatives, Orsellinic acid derivatives, Pulvinic acid derivatives, Anthraquinone derivatives, sctonemin, Mycosporine amino acids are of great importance [3] to pharmaceutics in Medicinal Industry [16]. This review article focused on the role of lichen compound in UV protection and its anticancer properties.
Lichens used in Traditional Medicine
The knowledge on importance of lichen compounds in traditional medicines is known for several centuries as it cures different ailments is presented in Table 1. [17,18]. However, initial contributions to medicinal uses of lichens were made during 1700-1800 BC from Evernia furfuracea (L.) mann. [18,19]. It has been evident from literature that these lichens display wide applications in Siddha, Ayurvedic and Unani systems for common ailments and cure many diseases like heart disease, wound, asthma, leprosy, bronchitis, stomach disorders, skin disease, etc., [20].
|
S. N |
Lichen Name |
Disease |
Country |
Reference |
|
1 |
Usnea ceratina Ach. |
Coughs, inflamed lungs, tuberculosis, headache, injury, snakebites |
China |
[115] |
|
2 |
Usnea sikkimensis Biswas sp. nov. |
Asthma, wound, hair strengthen, lung troubles, treat blisters |
India |
[116] |
|
3 |
Cladonia gracilis (L.) Willd. |
Dizziness, painful urination, nose bleeding, impetigo, pink eye, drink decoction |
China |
[115] |
|
4 |
Pertusaria pertusa (weigel) Tuck. |
Fever, kill worms, toothache |
Europe |
[117] |
|
5 |
Parmelia nepalense (Talyor) Hale |
Toothache, sore throat, |
Nepal |
[118] |
|
6 |
Thamnolia vermicularis (Schwartz) Ach. |
antiseptic |
India |
[119] |
|
7 |
Cetraria islandica (L.) Ach. |
Tuberculosis, chronic bronchitis, diarrhea, inflammation, ulcer, feed for deer and pigs |
Iceland |
[120] |
|
8 |
Xanthoparmelia conspersa (Ehrh. ex Ach.) Hale |
Syphilis eruptions, known and suspected snakebites, scarify bite |
South Africa |
[121] |
|
9 |
Parmotrema zollingeri (Hepp) Hale |
Used as medicine for children high fever and let the child smell the fumes |
Philippines |
[122] |
|
10 |
Punctelia borreri (Sm.) Krog |
Used for blurred vision, bleeding from uterus, bleeding from external injuries and swelling and chronic dermatitis. Drink decoction or apply powdered lichen to affected area |
China |
[115] |
|
11 |
Evernia divaricata (L.) Ach. |
Used for coughs, pneumonia, hepatitis, headaches, infection due to trauma, inflammation of the breasts, and snake bites. |
China |
[115] |
|
12 |
Ramalina capitata (Ach.) Nyl. |
Drunk as tea to relieve symptoms of asthma |
Spain |
[123] |
|
13 |
Lobaria pulmonaria (L.) Hoffm. |
It was mainly used in lung ailments (e.g tuberculosis, asthma, coughs, spitting blood) stimulant diarrhea, and stop bleeding. It was usually boiled water or milk and drunk or made ointment for external use. |
England, Germany, Sweden |
[124,125] |
|
14 |
Pseudocyphellaria aurata (Ach.) Vain. |
Used as tea to treat indigestion |
Madagascar |
[126] |
|
15 |
Peltigera aphthosa (L.) Wild. |
Used to improve digestion |
China |
[115] |
|
16 |
Cladonia subtenuis (Abbayes) Mattick |
Lichen used to relieve the pain of insect stings. |
USA |
[127] |
|
17 |
Bryoria fremontii (Tuck.) Brodo & D. Hawksw. |
Boiled and used as poultice for arthritis, Good for upset stomach, indigestion, and diarrhea |
USA |
[128] |
|
18 |
Usnea longissima Ach. |
Used to treating cancer, tuberculosis and ulcers, treating heal bone fractures. Washed, air-dried, soaked overnight in salted water, and placed over affected part |
Turkey, China, Indo-Tibetan Himalayas |
[129–131] |
|
19 |
Letharia vulpina (L.) Hue |
Used for Wolf poison. Pulverized, mixed with fat and flesh, wolf will die within 24 h of ingestion. |
Sweden |
[125] |
|
20 |
Heterodermia diademate (Taylor) D. D Awasthi |
Used for cuts and injuries |
India |
[132] |
|
21 |
Lobaria spp. (Schreber) Hoffm. |
Pulverized and made into a paste to cure skin diseases, Whole lichen used to treat indigestion. |
Bhutan, Tibet |
[133] |
|
22 |
Peltigera membranacea (Ach.) Nyl. |
Used as antiseptic and to stop bleeding. thalli made into paste and put on cuts |
Sikkim, India |
[134] |
|
23 |
Lasallia papulosa (Ach.) Llano |
Lichen used for urinary problems |
Canada |
[135] |
Table 1: Medicinal applications of Lichens.
Role of Lichen compounds in medicine and drug discovery
Recent advancement in the medical field has endowed a limited number of lichen compounds with amazing biological activities both in vitro and in vivo (Table 2). Usnic acid has a potential antimicrobial activity against Streptococcus mutants bacteria [21,22]. [23,24] The widely occurring lichen compounds such as diffractaic acid, norstictic acid, hypostictic acid, protocetraric, Barbatic acid were identified to inhibit the bacterial growth. In literature, most of the lichen compounds have been compiled which gives fair information regarding the anti-viral [25], antimicrobial property against both Gram positive and Gram negative bacteria [16]. Critical investigation on anti-prolifetative and cytotoxic activity of following lichen compounds parietin, atranorin, gyrophoric acid, usnic acid were carried out against HaCat, K-562, HEC-50, L1210, HeLa, A 2780, SK-BR-3, HCT-116, p53, HT-29, MCF-7 and proved its biological activities [26–29]. Several studies have confirmed antioxidant and pro-oxidant properties of lichen compounds which prevent oxidative damage [30–33]. It was noteworthy to identify that lichen compounds displayed antiviral activity against HIV [34,35], Papilloma virus, polyomavirus, influenza virus A (H1N1), polio virus, [25,36,37] protozoans [38,39]. The growth rate of cancer cells found arrested at s-phase or sub-G1 might probably be the reason for the cancer regulation control in lichen compound [40,41], Various lichen compounds such as usnic acid, atranorin, n-Butyl orsellinate, Lecanoric acid, 16-O-acetyl -leucotylic acid, diffractaic acid, divaricatic acid, retigeric acid, olivetoric acid, pannarin, gyrophoric acid , parietin are effective against following cancer cell lines such as A431, HCT-116, HL-60, HeLa, HaCaT, DU-145, HT-29, MCF-7, LNCaP, MM98, DU 145, T-47D, PC-3, H1299, K-563, A549, M14, and RCB-0461 [29,40–50]. Similarly, dichloromethane solvent fraction of following lichen acids from Heterodermia indica, Heterodermia microphylla, Heterodermia leucomela, Heterodermia podocarpa, Heterodermia diademata, Heterodermia punctifera and Heterodermia speciosa showed 80% mortality of cell lines in brine shrimp assay [51]. [52] showed ethyl acetate extract Heterodermia species had strong DPPH and TEAC (Trolox equivalents activity capacity) scavenging activity. Compounds like atranorin and Lobaric acid compounds extracted from Heterodermia obscurata were screened for analyzing immunomodulatory activity on respiratory burst of WBCs, isolated human PMN leukocytes and macrophages from murine using lucigenin-based chemiluminescence of luminol probes [53]. (Protocetraric acid exhibited cytotoxic effects against human melanoma and human colon carcinoma with an IC50 value of 58.68 µg/ml and 60.18 µg/ml respectively [54]. Protolichesterinic acid showed antitrypanosomal activity against Trypanosoma brucei with a MIC value of 12.5 µM. The molecular docking studies displayed its hydrophobicity property favors its free infiltration into pathogen cells [55]. Cytotoxicity analysis of protolichesterinic acid exhibited effective activity at the concentrations as high as 5 µM against human keratinocyte cell line [28]. Protolichesternic acid induces Cell apoptosis by inhibiting Hsp 70 protein expression and a redox-sensitive mechanism was indicated in LNCaP and DU-145 prostate cancer cell lines [56].
|
Patent No |
Lichen metabolites and application |
Reference |
|
US4424373A |
Preparation of secalonic acids used for innovative antibacterial agents |
[136] |
|
DE3229086A1 |
Cetraria islandica use in veterinary medicine for horses |
[137] |
|
US4556651A |
Secalonic acid derivatives as antitumor agents |
[138] |
|
US4536474A |
Tissue culture of lichens |
[139] |
|
US5169783A |
Increasing nucleation activity with lichens and fungi |
[140] |
|
US5260053A |
A Herbal deodorant composition for a key bactericide |
[141] |
|
EP0560227A2 |
Acetone extract of Nephromopsis ornata showing antiviral properties on Epstein-Barr virus |
[142] |
|
US5447721A |
Superoxide elimination activity of acetone extracts of Nephromopsis ornate and Vulpicida canadensis for cosmetic application |
[143] |
|
FR2756182B1 |
Crude extract of Cetraria islandica used to prevent and treat asthma |
[144] |
|
WO1999020793A1 |
Usnic acid and Vulpinic acid used to inhibit eukaryotic protein kinase for tentative and therapeutic uses. |
[145] |
|
US6811835B1 |
Lichen on rock camouflage pattern |
[146] |
|
US20030068294A1 |
Extract for Cetraria islandica used for veterinary medicine (ex. ear hygiene) |
[147] |
|
KR100453679B1 |
A hair color composition containing Tuckermannopsis ciliaris as a auxiliary component |
[148] |
|
CN1500520A |
Ethanol extract of lichen Parmelia tinctorum containing atranorin, salazinic acid and norstictic acid produced for antibiotics |
[149] |
|
US20040198815A1 |
Antimicrobial and Anticancer properties of Methyl-Beta-Orcinolcarboxylate from Lichen (Everniastrum cirrhatum) |
[150] |
|
WO2006125857A1 |
A polymer mixture established from lichen polysaccharides and other polymers for capsule coatings |
[151] |
|
WO2008077997A1 |
Cetraria islandica lichen based wood protection and impregnation product |
[152] |
|
RU2358750 C2 |
Pharmaceutical compositions based on barbate lichen (Usnea barbata) and common st john’s wort (Hypericum perforatum) and application thereop |
[153] |
|
US20120329868A1 |
A mixture of lichesterinic acid and protolichesterinic acid or their derivative compound used for stimulating pigmentation of skin and appendages |
[154] |
|
WO2012085559A1 |
Antibacterial and anti-acne skin care formulations contain usnic acid or usnate |
[155] |
|
JP2013253060A |
A process to produce a lichen extract for skin whitening agent |
[156] |
|
US20150105459A1 |
Lichesterinic acid and derivative compound to inhibit the skin pigments |
[157] |
|
US9139694B1 |
High temperature materials with low moisture uptake made from lichen metabolites |
[158] |
|
US9328202B1 |
High temperature materials with low moisture uptake made from lichen metabolites |
[159] |
|
US9539227B2 |
Pharmaceutical composition for the prevention or treatment of inflammatory diseases or immune diseases containing ramalin |
[160] |
|
US20190072494 A1 |
PH color indicator for use with agricultural compounds |
[161] |
Table 2: List of Patents in Lichen metabolites and its applications.
UV Radiation
The sun emits electromagnetic UV radiation. This radiation has different frequency and wavelength (Figure 1). They are shorter than visible light and longer than x rays. UV radiation is classified into three types. When the wavelength is between 100-280 nm these radiations are called shortwaves represented as UV C. When the wavelength is from 280 – 315 nm they are called a medium wave denoted as UV B which is partially absorbed by the ozone layer. When the radiation range is beyond 315 but less than 400 nm they are called longwave, regarded as UVA which reaches earth directly as ozone does not absorb UVA [57]. The radiation UV C is widespread in the ozone layer as the latter traps UVC from sunlight and hence it does not reach earth. Shortwave UV radiation damages the DNA.
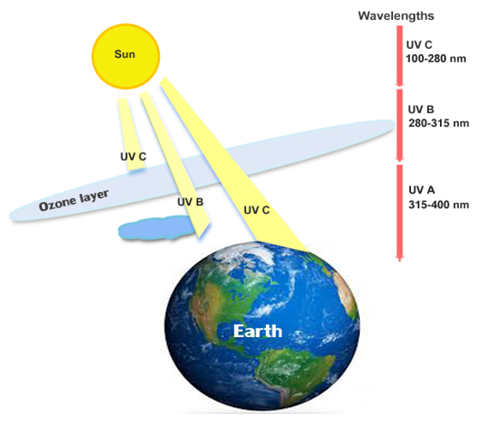
Figure 1: Different wavelengths of Ultraviolet radiation.
Sunburn is formed by the intense impact of UV A on skin surface and to cause skin cancer suntan. Some regions are normally devoid of ozone and therefore, the C radiation can be greatly found reaching through the ozone hole. Human retina, eye lens and cornea and were unable to see UV rays but rarely some children and young adults could see the UV rays [58,59]. UV radiations were visible to some mammals, insects and birds [60]. Over exposure of Ultraviolet radiation can cause not only sunburn, but other harmful effects such as skin cancer and eye damage [61].
UV A induces DNA damage and melanoma. Mutation alone accounts for 92% of the total UV exposed cells and melanoma cancer represents the effect of mutation [62]. The short wavelength range of UV C radiation causes contrary effects of mutation and carcinogenic damages [63].
Features of UV Radiation protecting chemicals
Aromatic and heteroaromatic rings are chief electron acceptor or donor regions in lichen compound and possessing UV absorbing capacity [64]. Among various UV absorbing compounds, lichen metabolite is represented by aromatic and heteroaromatic compounds such as benzene derivatives, biphenyl derivatives, indole derivatives, imidazole derivatives, purine derivatives, and naphthalene derivatives. Apart from these general views, unsaturated bonds (π) are significant and have been the specific receptor site in most UV absorption. Further, other compounds have heteroatoms like halogens, oxygen, sulfur or nitrogen and with unpaired electrons will excite to σ*, π* transitionspresence of heteroatom with a double bond affect absorption maximum [64]. UV B and UV A radiations are potential agents to irradiate molecules. Poly unsaturated hydrocarbons like β-carotene absorbs UV-visible light absorption maximum at 452 nm and high intensity (ε=15.2 104 L mol-1cm-1) [64]. Nguyen et al (2013) reported lichens have UV protectant metabolites such as depsidone derivatives, depside derivatives, xanthone derivatives, orsellinic derivatives, anthraquinone derivatives, scytonemin, pulvinic acid derivatives and mycosporine amino acid (Table 3). Lichen cyanobacteria produces non- aromatic compounds like Mycosporines and Mycoporine amino acids responsible for UV protection and play a vital photo-antioxidant role [65,66]. Some studies reported that high amount of phenolic compounds distributed mainly in the upper parts exposed under direct sunlight which effectively absorb UV B radiation [57,67]. Moreover, depsidones, depsides, dibenzofuranes, diphenyl ethers and chromones are representative agents to control UV B radiation while xanthones, pulvinic acid derivatives control the UV A radiation, absorb energy 10,000 L mol-1cm-1. The depside derivatives such as atranorin, barbatic acid [68] divaricatic acid, diffractaic acid, evernic acid, gyrophoric acid, isosphaeric acid and sphaephorin are reported to screen UV radiations [69,70].
The depsidones derivatives like pannarin, chloropannarin, salazinic acid, fumarprotocetraric acid, lobaric acid, variolaric acid, vicanicin, diploicin, scensidin, dechlorodiploicin, methyldiploicin are lichen compounds display UV B and UV A radiation absorbing properties [71–73]. Diploicin absorbs wavelengths of λ max= 320nm, dechlorodiploicin (λ max=315nm), and 4-O-methyldiploicin (λ max= 324nm) Millot reported [73].
Dechlorodiploicin has cyto-toxic effect on HaCaT cell lines [74]. Dibenzofurans derivatives, chromones, and xanthone compounds such as usnic acid, lepraric acid, placodiolic acid, epiphorellic acid, buellin and lichexanthone are reported to have the UV proof features. Usnic acid is the most common therapeutic lichen compound known to filter UV B radiation (λ max= 287nm) and ε=18 600 L mol-1 cm-1 [70]. There are few chromones isolated from lichens known to filter radiations.
Lepraric acid is one of the chromones, occurs in cortical and medullary layers of Roccella fuciformis [75]. The anthraquinone compounds influence UV B and blue light absorbing features in lichen species and. Similarly, anthraquinone and perylenequinone derivatives such as parietin [76], russulone, haematommone, isohypocrellin and elsinochrome respond to filter UV radiation [77]. Pulvinic acid derivatives display absorption of UV B and UV A are calycin, rhizocarpic acid, and vupinic acid [70,78]. The pulvinic acid derivative has to play a vital role in moderate UV protection but stable photo-protection [79]. These compounds are relatively lacking in energy transfer and therefore preventing DNA damage [71].
Mycosporines and Mycosporine amino acid (MAA) are polar, low molecular and water soluble compounds found in many marine lichens. The lichen symbiotic partner cyanobacteria synthesize mycosporines and MAA derivative such as mycosporine-glycine, mycosporine-taurine, mycosporine serinol, mycosporine hydroxyglutamicol, shinorine, mycosporine-2-glycine and euhalothece. These secondary compounds have high photostability and the ability to prevent the DNA damage caused by UV A and UV B radiation. However, a major limitation is its availability of low concentration of these compounds in marine organisms so isolation is difficult [80–82]. Scytonemin is a shikimic acid derivative synthesized in outer thallus of cyanobacterial lichens of some genera exposed under direct sunlight are Gonohymenia, Peltula and Collema species [83]. Scytonemin related compound dimethoxyscytonemin produced by cyanobacteria under uv exposed condition synthesize tetramethoxyscytonemin by the scytonemin reduction mechanism. These compounds have the ability to absorb in UV A, UV B and UV C radiation [84,85]. The carotenoid pigments of lichens have also displayed UV screening and photoprotective features [64,86]. The melanin, widely present in all species of fungi to humans, is a complex group of biological origin pigment to have UV protecting ability [87]. Accordingly, strong UV B absorbing melanin pigment has been harvested from lichen thallus of Cetraria islandica [88].
UV protectant mechanism
As a result of exposure of UVA (320-400 nm) radiation, skin cells produce ROS (reactive oxygen species) and reactive nitrogen species (RNS). The sun screen compounds are concerned with the interaction of antioxidants. These antioxidants are naturally found in lichen compounds. In fact, application of sunscreen paste having antioxidant substances which solves the misery associated with skin cancer as it protects the skin from formation of free radicals. Eventually, it protects skin from the toxic effects of ROS and RNS [89].
UV index
The Global Solar UV index is a reference action spectrum formulated for UV induced erythema on human skin defined by the International commission. It quantifies the amount of UV radiation which is relevant to induce effect for the horizontal surface. UV index is a unit less calculation represented by the formula [90].
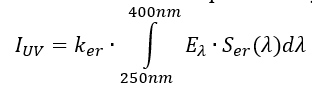
Eλ is solar spectral irradiance expressed in W∙/(m2∙nm1) at wavelength λ and dλ is the wavelength interval used in the summation. Serλ is an erythema reference action spectrum and Ker are the constant equal to 40 m2/w.
The UV Index can be determined through measurements or model calculations. Two measurement approaches can be considered: the first is using a spectro-radiometer to calculate the UV index using the above formula.
The second uses a broadband detector which calibrates to give the UV index directly. Prediction of the solar model that requires the input of the aerosol optical properties and total ozone. The total ozone is predicted using a regression model which calculates the input from ground-based ozone Spectro-radiometers or from satellites (Figure 2). A good cloud parameterization is also required unless only clear sky values will be reported [90].
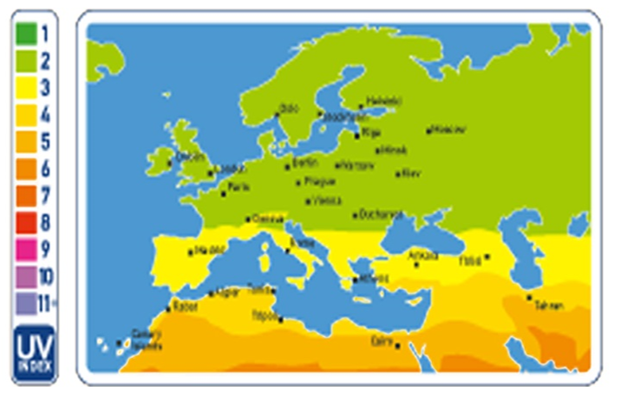
Figure 2: Global solar index Map [90].
Anticancer properties in lichens
Cancer is the deadliest and common disease leading to death around the world. Due to its medicinal significance, it has become a trend that many countries are on the lookout for agents from bacteria, marine microorganisms, fungi, plants, etc., and focusing on extraction of novel anticancer drugs.
Lichens belong to the plant kingdom. The application of lichen secondary metabolites as anti-tumour drugs dates back to the 1960s when the activity of lichen sugars against cancer cells was initially discovered [91]. An extensive research of many lichen compounds extracted on many different malignant cell lines showed a strong effect of cytotoxicity (Table 4) [28,92,93]. Structural slight modification of lichen compounds is found to increase cytotoxic potency of many lichen metabolites [43,50]. Various lichen compounds have been found to inhibit the growth of cancer cells at the Sor sub -G phase of the cell cycle [26,40,41]. The mechanism of cytotoxicity in cancer cell lines is caused by lichen metabolite induced apoptosis [26,92]. It has been evident from previous investigations reported that increase in the level of the Bax protein was associated with reduce in the Bcl-2 protein (Bax/Bcl-1:2 ratio) can induce the release of mitochondrial protein cytochrome c into the cytoplasm, as a result in the induction of caspase-3 which acts as an inducer of apoptosis [94,95]. Liches primary and secondary metabolites such as β-glucan and galactomannan have shown strong anticancer agents [96]. Recently cancer research studies indicated the use of lichen polysaccharides as immune-stimulants and they play a vital role against cancer cell lines [97,98]. Usnic acid evaluation of anticancer potency and associated with molecular alterations against human lung carcinoma A549 cell lines study reported that it inhibits cell growth and involving G0/G1 phase cell cycle arrest and induces cell death via mitochondrial membrane depolarization and induction of apoptosis in human lung carcinoma cell lines [99]. [48] Reported that usnic acid has anti-proliferative activity against the wild type TP53 nonfunctional breast cancer cell lines, and lung cancer cell line H1299 and is null for TP53. Lichen compound usnic acid as non-genotoxic anticancer agent studies in TP53 independently support to suppress tumor cells [48]. The cytotoxic mechanism of action of parietin, atranorin, gyrophoric acid and usnic acid was showed against A2780 and HT-29 cancer cells [100]. [101] Reported that usnic acid effectively inhibited in vivo angiogenesis in chicken embryos. Mouse tumor model usnic acid suppressed Bcap-37 breast tumor growth and angiogenesis. [26] evaluated sensitivity of various cancer cell line A2780,HeLa, SK-BR-3, HT-29, HCT-116, HCT-116, MCF-7, HL-60 for the anti-proliferative and cytotoxic effects of four lichen secondary compounds atranorin, parietin, gyrophoric acid and usnic acid. [43] Reported that usnic acid induced apoptosis on L1210 cell lines. Cetraria islandica lichen species produced a antiproliferative protolichesterinic acid against fourteen cancer cell lines and most of them showed IC50<10 µg/ml [102,103]. Pannarin and shpaerophorin are also reported to inhibit cell growth and induce apoptosis in human prostate carcinoma DU-145 and human melanoma M14 cell line [49,104]. Recently antiproliferative assays were carried out on A431 vulvar carcinoma, MM98 malignant mesothelioma cell line compared to HaCat keratinocytes with vulpinic, usnic, gyrophoric, salazinic, and evernic acids and confirmed the strong activity of usnic acid and showed interesting results about the disconnection of cell proliferation stimulation and mitosis inhibition [46]. (−)Usnic acids In vivo assays showed weak antitumoral effect against Lewis Lung carcinoma and P388 leukemia [29,46,105]. [46,106] reported salazinic acid had lower significant activity against MM98, HacaT, A431, HCT-8 MDA-MB435, and SF-295 cancer cells. According to [107], hypostictic acid had anticancer and antiproliferative activity against, MCF7, HT-29, HepG2, K562, NIH/3T3, PC-03, 786-0, B16-F10, cell lines. Hypostictic acid showed cytotoxic activity in following cell lines tested with GI50 value of 2.2-72.4 µm on B16-F10, K562 and 786-0 GI50 value of 2.2-13.8 and 14.2 µm. Lichen metabolite such as salazinic acid and hypostictic acid induced cell death by apoptosis at concentrations more than 25 µg/ml at 24 and 48 h of UV exposure. [108] Investigation reported that the cytotoxic activities of methyl orsellinate and tenuiorin extracted from Peltigera leucophlaebia lichen species tested on human breast T-47D, pancreatic PANC-1, and colon WIDR cancer cell lines, showed a mild to significant activity of tenuiorin on the pancreatic and colon cell lines, whereas methyl orsellinate had no effect. Depsidones and depsides extracted from Antarctic lichens were investigated with colchicine in in vitro cell lines of lymphocytes or with usnic acid for their apoptotic and cytotoxic activity on liver cell lines [109]. Lichen glucans did not show cytotoxic actions with the IC50 values on cancer cells as exemplified by the galactomannose substituted glugan extracted from Cladonia furcate IC50 500-800 µg/ml. Apoptosis induction and a telomerase activity diminution demonstrated their potential in anticancer adjuvants [110]. According to [111] physodic acid showed high activity with the IC50 value of 26.7 µm against melanoma cancer cells. [112] Reported that depsidones derivative such as protocetraric acid, norstictic, and psoromic acid and depside derivatives of divaricatic and perlatolic acids showed strong activity against UACC-62 melanoma cells and 3T3 normal cells.
Molecular mechanisms and anticancer activity of Lichen metabolite atranorin exhibited antitumorigenic activity in a mouse xenograft tumor. Further investigation revealed that nuclear Ki-67 level reduction and expression of nuclear protein occurred in cancer cells in all phases of the active cell cycle [113]. [114] Vulpinic acid showed cytotoxicity at a concentration with the IC50 value is 23.8 µm against HepG2 cancer cell line and this study reported that vulpinic acid could be used as a novel drug source in the pharmaceutical industry.
Conclusion
Activities of lichen metabolites are illustrated in-detail and at this juncture, it is realized that these lichen compounds are the least explored agents among anticytotoxic sunscreen drugs. The challenge here is lack of rapid in vitro culture methods in undertaking the commercial production of lichen compounds and rediscovery of effective drugs to cure the potential disease cancer. The study targeted the deleterious effect of UV radiation in western countries and importance of UV proof sunscreen lichen compounds. However, in the current decade, most of the advancements in microbiology not only solves the cultivation problem but also helps in production of lichen compounds with great success. There are many UV screening compounds produced by various lichen species. They are grouped under poly functionalized aromatic compounds. Atranorin, calycin, pannarin, parietin and usnic acids were the most investigated UV screen compounds derived from lichens. Based on the review, it is concluded that these lichen compounds exhibited strong in vitro and in vivo anticancer activities and hence, it can be used as a novel sunscreen drug source in the drug industry.
Acknowledgement
Authors express their gratitude to Dr. A. Rajendran, Prof. & Head, Department of Botany, Bharathiar University, Coimbatore for providing excellent help throughout this research work. We are thankful to UGC SAP and DST FIST, Government of India for financial support to carry out this study.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Zhao Y, Wang M, Xu B (2020) A comprehensive review on secondary metabolites and health-promoting effects of edible lichen. Journal of Functional Foods: 104283.
- Hei Y, Zhang H, Tan N, Zhou Y, Wei X, et al. (2021) Antimicrobial activity and biosynthetic potential of cultivable actinomycetes associated with Lichen symbiosis from Qinghai-Tibet Plateau. Microbiological Research 244: 126652.
- Roy S, Soni P (2021) Lichen as nature’s basket full of bioactive compounds. In New and Future Developments in Microbial Biotechnology and Bioengineering (pp. 117-130). Elsevier.
- Fahselt D (1994) Secondary biochemistry of lichens. Symbiosis: USA.
- Hamada N, Miyagawa H, Miyawaki H, Inoue M (1996) Lichen Substances in Mycobionts of Crustose Lichens Cultured on Media with Extra Sucrose. The Bryologist 99: 71–74.
- Kon Y, Kashiwadani H, Wardlaw JH, Elix JA (1997) Effects of culture conditions on dibenzofuran production by cultured mycobionts of lichens. Symbiosis: USA.
- Stocker-Wörgötter E, Elix JA (2002) Secondary chemistry of cultured mycobionts: formation of a complete chemosyndrome by the lichen fungus of Lobaria spathulata. The Lichenologist 34: 351–359.
- Fazio AT, Bertoni MD, Adler MT, Ruiz LB, Rosso ML, et al. (2009) Culture studies on the mycobiont isolated from Parmotrema reticulatum (Taylor) Choisy: metabolite production under different conditions. Mycol. Prog 8: 359.
- Suzuki MT, Parrot D, Berg G, Grube M, Tomasi S (2016) Lichens as natural sources of biotechnologically relevant bacteria. Appl. Microbiol. Biotechnol 100: 583–595.
- Gallagher RP, Lee TK, Bajdik CD, Borugian M (2010) Ultraviolet radiation. Chronic Dis. Can 1: 51–68.
- Khazaei Z, Ghorat F, Jarrahi AM, Adineh HA, Sohrabivafa M, et al. (2019) Global incidence and mortality of skin cancer by histological subtype and its relationship with the Human Development Index (HDI); an ecology study in 2018. World Cancer Res. J 6: e1265.
- Kim HY, Jin H, Bae J, Choi HK (2019) Metabolic and lipidomic investigation of the antiproliferative effects of coronatine against human melanoma cells. Sci. Rep 9: 3140.
- World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.14. ISBN 978-9283204299.
- Molnár K, Farkas E (2014) Current Results on Biological Activities of Lichen Secondary Metabolites: a Review. Z Für Naturforschung C 65: 157–173.
- Shrestha G, St. Clair LL (2013) Lichens: a promising source of antibiotic and anticancer drugs. Phytochem. Rev 12: 229–244.
- Ingólfsdóttir K (2002) Usnic acid. Phytochemistry 61: 729–736.
- Linnaeus C, Flora Lapponica. apud Salomonem Schouten: Amstelaedami, 1737.
- Malhotra S, Subban R, Singh A (2008) Lichens-role in traditional medicine and drug discovery. Internet. J. Altern. Med 5: 1–5.
- Launert E (1981) Edible and Medicinal Plants: Covers Plants in Europe. Hamlyn Publishing Group Ltd., London.
- Shukla V, Joshi GP, Rawat MSM (2010) Lichens as a potential natural source of bioactive compounds: a review. Phytochem. Rev 9: 303–314.
- Ghione M, Parrello D, Grasso L (1988) Usnic acid revisited, its activity on oral flora. Chemioter Int. J. Mediterr. Soc. Chemother 7: 302–305.
- Lauterwein M, Oethinger M, Belsner K, Peters T, Marre R (1995) In vitro activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid, and (-)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob. Agents Chemother 39: 2541–2543.
- Honda NK, Pavan FR, Coelho RG, de Andrade Leite SR, Micheletti AC, et al. Antimycobacterial activity of lichen substances. Phytomedicine 17: 328–332.
- Martins MCB, Lima MJG, Silva FP, Azevedo-Ximenes E, Silva NH, et al. (2010) Cladia aggregata (lichen) from Brazilian Northeast: Chemical Characterization and Antimicrobial Activity. Braz. Arch. Biol. Technol 53: 115–122.
- Perry NB, Benn MH, Brennan NJ, Burgess EJ, Ellis G, et al. (1999) Antimicrobial, Antiviral and Cytotoxic Activity of New Zealand Lichens. The Lichenologist 31: 627–636.
- Bačkorová M, Bačkor M, Mikeš J, Jendželovský R, Fedoročko P (2011) Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. In Vitro 25: 37–44.
- Kristmundsdóttir T, Aradóttir HA, Ingólfsdóttir K, Ögmundsdóttir HM (2002) Solubilization of the lichen metabolite (+)-usnic acid for testing in tissue culture. J. Pharm. Pharmacol 54: 1447–1452.
- Kumar KCS, Müller K (1999) Lichen Metabolites. 2. Antiproliferative and Cytotoxic Activity of Gyrophoric, Usnic, and Diffractaic Acid on Human Keratinocyte Growth. J. Nat. Prod 62: 821–823.
- Takai M, Uehara Y, Beisler JA (1979) Usnic acid derivatives as potential antineoplastic agents. J. Med. Chem 22: 1380–1384.
- Halici M, Odabasoglu F, Suleyman H, Cakir A, Aslan A, et al. (2005) Effects of water extract of Usnea longissima on antioxidant enzyme activity and mucosal damage caused by indomethacin in rats. Phytomedicine 12: 656–662.
- Halliwell B (2008) Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem. Biophys 476: 107–112.
- Rabelo TK, Zeidán-Chuliá F, Vasques LM, dos Santos JPA, da Rocha RF, et al. (2012) Redox characterization of usnic acid and its cytotoxic effect on human neuron-like cells (SH-SY5Y). Toxicol. In Vitro 26: 304–314.
- Rice-evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB (1995) The Relative Antioxidant Activities of Plant-Derived Polyphenolic Flavonoids. Free Radic. Res 22: 375–383.
- Nakanishi T, Murata H, Inatomi Y, Inada A, Murata J (1998) Screening of Anti-HIV-1 Activity of North American Plants.: Anti-HIV-1 Activities of Plant extracts, and Active Components of Lethalia vulpina (L.) Hue. Nat. Med 52: 521–526.
- Neamati N, Hong H, Mazumder A, Wang S, Sunder S, et al. (1997) Depsides and Depsidones as Inhibitors of HIV-1 Integrase: Discovery of Novel Inhibitors through 3D Database Searching. J. Med. Chem 40: 942–951.
- Campanella L, Delfini M, Ercole P, Iacoangeli A, Risuleo G (2002) Molecular characterization and action of usnic acid: a drug that inhibits proliferation of mouse polyomavirus in vitro and whose main target is RNA transcription. Biochimie 84: 329–334.
- Sokolov DN, Zarubaev VV, Shtro AA, Polovinka MP, Luzina OA, et al. (2012) Anti-viral activity of (−)- and (+)-usnic acids and their derivatives against influenza virus A(H1N1)2009. Bioorg. Med. Chem. Lett 22: 7060–7064.
- De Carvalho EAB, Andrade PP, Silva NH, Pereira EC, Figueiredo RCBQ (2005) Effect of usnic acid from the lichen Cladonia substellata on Trypanosoma cruzi in vitro: an ultrastructural study. Micron 36: 155–161.
- Schmeda‐Hirschmann G, Tapia A, Lima B, Pertino M, Sortino M, et al. (2008) A new antifungal and antiprotozoal depside from the andean lichen Protousnea poeppigii. Phytother. Res 22: 349–355.
- Liu H, Liu Y, Liu Y, Xu A, Young CYF, et al. (2010) A novel anticancer agent, retigeric acid B, displays proliferation inhibition, S phase arrest and apoptosis activation in human prostate cancer cells. Chem. Biol. Interact 188: 598–606.
- Ren MR, Hur JS, Kim JY, Park KW, Park SC, et al. (2009) Anti-proliferative effects of Lethariella zahlbruckneri extracts in human HT-29 human colon cancer cells. Food Chem. Toxicol 47: 2157–2162.
- Ari F, Celikler S, Oran S, Balikci N, Ozturk S, et al. (2014) Genotoxic, cytotoxic, and apoptotic effects of Hypogymnia physodes (L.) Nyl. on breast cancer cells. Environ. Toxicol 29: 804–813.
- Bazin MA, Lamer ACL, Delcros JG, Rouaud I, Uriac P, et al. (2008) Synthesis and cytotoxic activities of usnic acid derivatives. Bioorg. Med. Chem 16: 6860–6866.
- Bogo D, Matos M de FC, Honda NK, Pontes EC, Oguma PM, et al. (2010) In vitro Antitumour Activity of Orsellinates. Z Für Naturforschung C 65: 43–48.
- Brisdelli F, Perilli M, Sellitri D, Piovano M, Garbarino JA, et al. (2012) Cytotoxic Activity and Antioxidant Capacity of Purified Lichen Metabolites: An In Vitro Study. Phytother. Res 27: 431–437.
- Burlando B, Ranzato E, Volante A, Appendino G, Pollastro F, et al. (2009) Antiproliferative Effects on Tumour Cells and Promotion of Keratinocyte Wound Healing by Different Lichen Compounds. Planta Med 75: 607–613.
- Koparal AT, Tüylü BA, Türk H (2006) In vitro cytotoxic activities of (+)-usnic acid and (−)-usnic acid on V79, A549, and human lymphocyte cells and their non-genotoxicity on human lymphocytes. Nat. Prod. Res 20: 1300–1307.
- Mayer M, O’Neill MA, Murray KE, Santos-Magalhães NS, Carneiro-Leão AMA, et al. (2005) Usnic acid: a non-genotoxic compound with anti-cancer properties. Anticancer Drugs 16: 805.
- Russo A, Piovano M, Lombardo L, Vanella L, Cardile V, et al. (2006) Pannarin inhibits cell growth and induces cell death in human prostate carcinoma DU-145 cells. Anticancer Drugs 17: 1163.
- Tokiwano T, Satoh H, Obara T, Hirato H, Yoshizawa Y, et al. (2009) Lichen Substance as an Antiproliferative Compound against HL-60 Human Leukemia Cells: 16-O-Acetyl-leucotylic Acid Isolated from Myelochroa aurulenta. Biosci. Biotechnol. Biochem 73: 2525–2527.
- Jha BN, Shrestha M, Pandey DP, Bhattarai T, Bhattarai HD, et al. (2017) Investigation of antioxidant, antimicrobial and toxicity activities of lichens from high altitude regions of Nepal. BMC Complement Altern. Med 17: 282.
- Behera BC, Mangesh VM, Subhash BG (2016) Anti-lipoxygenase, Radical Scavenging and Antimicrobial Activities of Lichen Species of Genus Heterodermia (Physciaceae). Bot. Pacifica J. Plant Sci. Conserv 5: 79–85.
- Thadhani VM, Mesaik MA, Asif M, Karunaratne V, Choudhary IM (2015) Immunomodulatory activities of some common lichen metabolites. Int. J. Pharm. Pharm. Sci 7: 144–147.
- Manojlović N, Ranković B, Kosanić M, Vasiljević P, Stanojković T (2012) Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine 19: 1166–1172.
- Ogbaji Igoli J, Irvine Gray A, Jean Clements C, Kantheti P, Kumar Singla R (2014) Antitrypanosomal activity & docking studies of isolated constituents from the lichen Cetraria islandica: possibly multifunctional scaffolds. Curr. Top. Med. Chem 14: 1014–1021.
- Russo A, Caggia S, Piovano M, Garbarino J, Cardile V (2012) Effect of vicanicin and protolichesterinic acid on human prostate cancer cells: Role of Hsp70 protein. Chem. Biol. Interact 195: 1–10.
- Buffoni Hall RS, Bornman JF, Björn LO (2002) UV-induced changes in pigment content and light penetration in the fruticose lichen Cladonia arbuscula ssp. mitis. J. Photochem. Photobiol. B 66: 13–20.
- Lynch DK, Livingston WC, Livingston W (2001) Color and light in nature. Cambridge University Press.
- Mainster MA (2006) Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br. J. Ophthalmol 90: 784–792.
- Hunt DM, Carvalho LS, Cowing JA, Davies WL (2009) Evolution and spectral tuning of visual pigments in birds and mammals. Philos. Trans. R. Soc. B. Biol. Sci 364: 2941–2955.
- Meyer-Rochow VB (2000) Risks, especially for the eye, emanating from the rise of solar UV-radiation in the Arctic and Antarctic regions. Int. J. Circumpolar Health 59: 38–51.
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, et al. (2002) Mutations of the BRAF gene in human cancer. Nature 417: 949–954.
- Hogan CM (2011) Sunlight. eds. P Saundry C Clevel Encycl Earth.
- Cockell CS, Knowland J (1999) Ultraviolet radiation screening compounds. Biol. Rev 74: 311–345.
- Aspée A, Aliaga C, Scaiano JC (2007) Transient Enol Isomers of Dibenzoylmethane and Avobenzone as Efficient Hydrogen Donors toward a Nitroxide Pre-fluorescent Probe†. Photochem. Photobiol 83: 481–485.
- Huneck S, Yoshimura I (1996) Identification of Lichen Substances. In: Huneck S, Yoshimura I, editors. Identification of Lichen Substances. Springer: Berlin, Heidelberg: 11–123.
- Swanson A, Fahselt D (1997) Effects of ultraviolet on polyphenolics of Umbilicaria americana. Can. J. Bot 75: 284–289.
- Begora M, Fahelt D (2001) Photolability of secondary compounds in some lichen species. Symbiosis 31: 3–22.
- Fernández E, Quilhot W, González I, Hidalgo ME, Molina X, et al. (1996) Lichen metabolites as UVB filters: Lichen metabolites show photoprotector capacity. Cosmet. Toilet 111: 69–74.
- Lohézic-Le Dévéhat F, Legouin B, Couteau C, Boustie J, Coiffard L (2013) Lichenic extracts and metabolites as UV filters. J. Photochem. Photobiol. B 120: 17–28.
- Boehm F, Clarke K, Edge R, Fernandez E, Navaratnam S, et al. (2009) Lichens – Photophysical studies of potential new sunscreens. J. Photochem. Photobiol. B 95: 40–45.
- Hidalgo ME, Bascuñan L, Quilhot W, Fernández E, Rubio C (2005) Spectroscopic and Photochemical Properties of the Lichen Compound Lobaric Acid. Photochem. Photobiol 81: 1447–1449.
- Millot M, Di Meo F, Tomasi S, Boustie J, Trouillas P (2012) Photoprotective capacities of lichen metabolites: A joint theoretical and experimental study. J. Photochem. Photobiol. B 111: 17–26.
- Millot M, Tomasi S, Studzinska E, Rouaud I, Boustie J (2009) Cytotoxic Constituents of the Lichen Diploicia canescens. J. Nat. Prod 72: 2177–2180.
- Aberhart DJ, Overton KH, Huneck S (1969) Studies on lichen substances. Part LXII. Aromatic constituents of the lichen Roccella fuciformis DC. A revised structure for lepraric acid. J. Chem. Soc. C Org: 704–707.
- Vráblíková H, McEvoy M, Solhaug KA, Barták M, Gauslaa Y (2006) Annual variation in photoacclimation and photoprotection of the photobiont in the foliose lichen Xanthoria parietina. J. Photochem. Photobiol. B 83: 151–162.
- Mulrooney CA, O’Brien EM, Morgan BJ, Kozlowski MC (2012) Perylenequinones: Isolation, Synthesis, and Biological Activity. Eur. J. Org. Chem: 3887–3904.
- Hidalgo ME, Fernández E, Ponce M, Rubio C, Quilhot W (2002) Photophysical, photochemical, and thermodynamic properties of shikimic acid derivatives: calycin and rhizocarpic acid (lichens). J. Photochem. Photobiol. B 66: 213–217.
- Rancan F, Rosan S, Boehm K, Fernández E, Hidalgo ME, et al. (2002) Protection against UVB irradiation by natural filters extracted from lichens. J. Photochem. Photobiol. B 68: 133–139.
- Pattanaik B, Roleda MY, Schumann R, Karsten U (2008) Isolate-specific effects of ultraviolet radiation on photosynthesis, growth and mycosporine-like amino acids in the microbial mat-forming cyanobacterium Microcoleus chthonoplastes. Planta 227: 907–916.
- Singh SP, Sinha RP, Klisch M, Häder DP (2008) Mycosporine-like amino acids (MAAs) profile of a rice-field cyanobacterium Anabaena doliolum as influenced by PAR and UVR. Planta 229: 225–233.
- White JD, Cammack JH, Sakuma K, Rewcastle GW, Widener RK (1995) Transformations of quinic acid. asymmetric synthesis and absolute configuration of Mycosporin I and Mycosporin-gly. J. Org. Chem 60: 3600–3611.
- Büdel B, Karsten U, Garcia-Pichel F (1997) Ultraviolet-absorbing scytonemin and mycosporine-like amino acid derivatives in exposed, rock-inhabiting cyanobacterial lichens. Oecologia 112: 165–172.
- Fleming ED, Castenholz RW (2007) Effects of periodic desiccation on the synthesis of the UV-screening compound, scytonemin, in cyanobacteria. Environ. Microbiol 9: 1448–1455.
- Rath J, Mandal S, Adhikary SP (2012) Salinity induced synthesis of UV-screening compound scytonemin in the cyanobacterium Lyngbya aestuarii. J. Photochem. Photobiol. B 115: 5–8.
- Stange C, Flores C (2012) Carotenoids and photosynthesis-regulation of carotenoid biosyntesis by photoreceptors. Adv. Photosynth. Fundam. Asp Rijekia Croat InTech: 77–96.
- Solhaug KA, Gauslaa Y (2012) Secondary Lichen Compounds as Protection Against Excess Solar Radiation and Herbivores. Progress in Botany 73. Springer: Berlin, Heidelberg 283–304.
- Nybakken L, Solhaug KA, Bilger W, Gauslaa Y (2004) The lichens Xanthoria elegans and Cetraria islandica maintain a high protection against UV-B radiation in Arctic habitats. Oecologia 140: 211–216.
- Rojas JL, Diaz-Santos M Valencia-Islas NA (2015) Metabolites with Antioxidant and Photo-Protective Properties from Usnea roccellina Motyka, a Lichen from Colombian Andes. UK J, Pharm. Biosci 3: 18.
- World Health Organization. Global solar UV index: a practical guide. WHO, 1995.
- Fukuoka F, Nakanishi M, Shibata S, Nishikawa Y, Takeda T, et al. (1968) Polysaccharides in lichens and fungi. GANN Jpn J. Cancer. Res 59: 421–432.
- Bézivin C, Tomasi S, Rouaud I, Delcros JG, Boustie J (2004) Cytotoxic Activity of Compounds from the Lichen: Cladonia convoluta. Planta Med 70: 874–877.
- Zeytinoglu H, Incesu Z, Tuylu BA, Turk AO, Barutca B (2008) Determination of genotoxic, antigenotoxic and cytotoxic potential of the extract from lichen Cetraria aculeata (Schreb.) Fr. in vitro. Phytother. Res 22: 118–123.
- Nishikawa Y, Ohki K, Takahashi K, Kurono G, Fukuoka F, et al. (1974) Studies on the water soluble constituents of lichens. II. Antitumor polysaccharides of Lasallia, Usnea, and Cladonia species. Chem. Pharm. Bull. (Tokyo) 22: 2692–2702.
- Nishikawa Y, Ohno H (1981) Studies on the water-soluble constituents of lichens. IV. Effect of antitumor lichen-glucans and related derivatives on the phagocytic activity of the reticuloendothelial system in mice. Chem. Pharm, Bull. (Tokyo) 29: 3407–3410.
- Watanabe M, Iwai K, Shibata S, Takahashi K, Narui T, et al. (1986) Purification and Characterization of Mouse α1-Acid Glycoprotein and Its Possible Role in the Antitumor Activity of Some Lichin Polysaccharides. Chem. Pharm. Bull. (Tokyo) 34: 2532–2541.
- Cordeiro LMC, de Oliveira SM, Buchi DF, Iacomini M (2008) Galactofuranose-rich heteropolysaccharide from Trebouxia sp., photobiont of the lichen Ramalina gracilis and its effect on macrophage activation. Int. J. Biol. Macromol 42: 436–440.
- Karunaratne DN, Jayalal RGU, Karunaratne V (2012) Lichen polysaccharides. Complex World Polysacch: 215–226.
- Singh N, Nambiar D, Kale RK, Singh RP (2013) Usnic Acid Inhibits Growth and Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma A549 Cells. Nutr. Cancer 65: 36–43.
- Bačkorová M, Jendželovský R, Kello M, Bačkor M, Mikeš J, et al. (2012) Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol. In Vitro 26: 462–468.
- Song Y, Dai F, Zhai D, Dong Y, Zhang J, et al. (2012) Usnic acid inhibits breast tumor angiogenesis and growth by suppressing VEGFR2-mediated AKT and ERK1/2 signaling pathways. Angiogenesis 15: 421–432.
- Haraldsdóttir S, Guðlaugsdóttir E, Ingólfsdóttir K, Ögmundsdóttir HM (2004) Anti-Proliferative Effects of Lichen-Derived Lipoxygenase Inhibitors on Twelve Human Cancer Cell Lines of Different Tissue Origin in vitro. Planta Med 70: 1098–1100.
- Ögmundsdóttir HM, Zoëga GM, Gissurarson SR, Ingólfsdóttir K (1998) Natural Products: Anti-proliferative Effects of Lichen-derived Inhibitors of 5-Lipoxygenase on Malignant Cell-lines and Mitogen-stimulated Lymphocytes. J. Pharm. Pharmacol 50: 107–115.
- Russo A, Piovano M, Lombardo L, Garbarino J, Cardile V (2008) Lichen metabolites prevent UV light and nitric oxide-mediated plasmid DNA damage and induce apoptosis in human melanoma cells. Life Sci 83: 468–474.
- Morris Kupchan S, Kopperman HL (1975) l-Usnic acid: tumor inhibitor isolated from lichens. Cell Mol. Life Sci 31: 625–625.
- Micheletti AC, Beatriz A, Lima DP de, Honda NK, Pessoa C. do Ó, et al. (2009) Constituintes químicos de Parmotrema lichexanthonicum Eliasaro & Adler – isolamento, modificações estruturais e avaliação das atividades antibiótica e citotóxica. Chemical constituents of parmotrema lichexanthonicum Eliasaro & Adler – isolation, structure modification and evaluation of antibiotic and cytotoxic activities.
- Alexandrino CAF, Honda NK, Matos M. de F.C, Portugal LC, Souza PRB, et al. (2019) Antitumor effect of depsidones from lichens on tumor cell lines and experimental murine melanoma. Rev. Bras. Farmacogn 29: 449–456.
- Ingólfsdóttir K, Gudmundsdóttir GF, Ögmundsdóttir HM, Paulus K, Haraldsdóttir S, et al. (2002) Effects of tenuiorin and methyl orsellinate from the lichen Peltigera leucophlebia on 5-/15-lipoxygenases and proliferation of malignant cell lines in vitro. Phytomedicine 9: 654–658.
- Correché ER, Enriz RD, Piovano M, Garbarino J, Gómez-Lechón MJ (2004) Cytotoxic and apoptotic effects on hepatocytes of secondary metabolites obtained from lichens. Altern. Lab. Anim 32: 605–615.
- Lin X, Cai YJ, Li ZX, Chen Q, Liu ZL, et al. (2003) Structure determination, apoptosis induction, and telomerase inhibition of CFP-2, a novel lichenin from Cladonia furcata. Biochim. Biophys Acta BBA – Gen Subj 1622: 99–108.
- Cardile V, Graziano ACE, Avola R, Piovano M, Russo A (2017) Potential anticancer activity of lichen secondary metabolite physodic acid. Chem. Biol. Interact 263: 36–45.
- Brandão LFG, Alcantara GB, Matos M. de FC, Bogo D, dos Santos Freitas D, et al. (2012) Cytotoxic evaluation of phenolic compounds from lichens against melanoma cells. Chem. Pharm. Bull. (Tokyo): c12–00739.
- Zhou R, Yang Y, Park SY, Nguyen TT, Seo YW, et al. (2017) The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis. Sci. Rep 7: 8136.
- Kılıc N, Derici MK, Buyuk I, Aydın SS, Aras S, et al. (2018) Evaluation of in vitro Anticancer Activity of Vulpinic Acid and its Apoptotic Potential Using Gene Expression and Protein Analysis. Indian J. Pharm. Educ. Res 52: 626–634.
- Crawford SD (2015) Lichens Used in Traditional Medicine. In: Ranković B, editor. Lichen Secondary Metabolites. Springer International Publishing: Cham: 27–80.
- Pradhan BK, Badola HK (2008) Ethnomedicinal plant use by Lepcha tribe of Dzongu valley, bordering Khangchendzonga Biosphere Reserve, in North Sikkim, India. J. Ethnobiol. Ethnomedicine 4: 22.
- Lebail BEF (1853) Des lichens: considérés sous le point de vue économique, médical et physiologique (nutrition). Rignoux.
- Kumar SK, Banskota AH, Manandhar MD (1996) Isolation and identification of some chemical constituents of Parmelia nepalensis. Planta Med 62: 93–94.
- Negi HR, Kareem A (1996) Lichens: The unsung heroes. Amrut 1:3–6.
- Zemlinskii SE (1958) Medicinal Plants of the USSR. State Med Lit Press Mosc.
- Smith A (1895) A contribution to South African materia medica: Chiefly from plants in use among the natives. JC Juta.
- Madulid DA, Gaerlan FJM, Romero EM, Agoo EMG (1989) Ethnopharmacological study of the Ati tribe in Nagpana, Barotac Viejo, Iloilo. Acta Manil 38: 25–40.
- Agelet A, Vallès J (2003) Studies on pharmaceutical ethnobotany in the region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part III. Medicinal uses of non-vascular plants. J. Ethnopharmacol 84: 229–234.
- De Crespigny RC, Hutchinson HG (1895) The new forest: its traditions, inhabitants and customs. John Murray.
- Withering W (1801) A Systematic Arrangement of British Plants; with an Easy Introduction to the Study of Botany, ed. 4, vol. 3. Musci H Baldwin Son Lond.
- Sharnoff SD (1997) Lichens and people.
- Garrett JT (2003) The Cherokee herbal: native plant medicine from the four directions. Simon and Schuster.
- Hunn ES, Selam J (1991) Nch’i-wana,” the big river”: Mid-Columbia Indians and their land. University of Washington Press.
- Odabasoglu F, Cakir A, Suleyman H, Aslan A Bayir Y, et al. (2006) Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol 103: 59–65.
- Sharma GK (1997) Ethnomedicinal Flora: Ayurvedic System of Medicine In. J. Tenn. Acad. Sci 72: 53–55.
- Yazici K, Aslan A (2003) Lichens from the regions of Gümüshane, Erzincan and Bayburt (Turkey). Cryptogam Mycol. Fr.
- Chandra S, Singh A (1971) A lichen crude drug (chharila) from India. J. Res. Indian Med 6: 209–215.
- Ju Y, Zhuo J, Liu B, Long C (2013) Eating from the wild: diversity of wild edible plants used by Tibetans in Shangri-la region, Yunnan, China. J. Ethnobiol. Ethnomedicine 9: 28.
- Saklani A, Jain SK (1994) Cross-cultural ethnobotany of northeast India. Deep publications.
- Uprety Y, Asselin H Dhakal A, Julien N (2012) Traditional use of medicinal plants in the boreal forest of Canada: review and perspectives. J. Ethnobiol. Ethnomedicine 8: 7.
- Kurobane I, Vining LC, McInnes AG (2019) Secalonic acids.
- Wichert B DI (1984) Medicine for coughs and other disorders of the respiratory organs of horses, and feedstuff for horses produced using the same.
- Shibukawa M, Shibuya C, Ishii K (1985) Secalonic acid derivatives as antitumor agents.
- Yamamoto Y, Mizuguchi R, Yamada Y (1985) Tissue culture of lichens.
- Kieft TL (1992) Increasing nucleation activity with lichens and fungi.
- Chappell KC, Scheeler PA, Rittershaus G (1993) Herbal deodorant.
- Miura Y, Higuchi M, Kinoshita Y, Yamamoto Y, Ohigashi H, et al. (1993) Epstein-barr virus activation inhibitor from lichens.
- Miura Y, Higuchi M, Knoshita Y, Yamamoto Y, Ohigashi H, et al. (1995) Superoxide eliminating agent.
- Etienne. Using the Iceland lichen to the bottom of treatment of bronchial asthma Classifications. 1998.
- Davies JE, Waters B, Saxena G (1999) Method for inhibiting eukaryotic protein kinases.
- Parietti MI (2004) Lichen on rock camouflage pattern.
- Cabrera AL, Beguer JH (2003) Preparation for veterinary use.
- Jin (2004) Composition for hair coloring samples.
- Yao, et al. (2004) Lichen bacteriophage and its production method thereof.
- Khanuja S, Tiruppadiripuliyur R, Gupta V, Chand P, Garg A, et al. (2004) Antimicrobial and anticancer properties of methyl-beta-orcinolcarboxylate from lichen (Everniastrum cirrhatum.
- Reijonen MT (2006) Composition and manufacturing process of cetraria islandica based polymer blend.
- Reijonen MT (2008) Cetraria islandica based wood protection and impregnation product.
- Kristof et al. (2009) Pharmaceutical compositions based on barbate lichen (usnea barbata) and common st john’s wort (hypericum perforatum) and application thereof.
- Boustie J, Galibert-Anne MD, Lohezic-le-Devehat F, Chollet-Krugler M, Tomasi S (2012) Paraconic acids as pigmentation activators.
- Eady EA, Fitzgerald DJ (2012) Antibacterial or anti-acne formulations containing usnic acid or an usnate and a metal salt.
- Takashi et al. (2013) Process for producing extract from lichen belonging to genus usnea.
- Boustie J, Galibert-Anne MD, Devehat FL, Chollet-Krugler M, Tomasi S, et al. (2015) Lichesterinic acid and the derivatives of same as pigmentation inhibitors.
- Davis MC (2015) High temperature materials with low moisture uptake made from lichen metabolites.
- Davis MC (2016) High temperature materials with low moisture uptake made from lichen metabolites.
- Yim JH, Kim IC, Lee SG, Kim DK, HAN SJ, et al. (2017) Pharmaceutical composition for the prevention or treatment of inflammatory diseases or immune diseases containing ramalin.
- Bloomberg MD, Houston-McMillan MS (2019) Ph colour indicator for use with agricultural compounds.
- Bézivin C, Tomasi S, Lohézic-Le Dévéhat F, Boustie J (2003) Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 10: 499–503.
- Triggiani D, Ceccarelli D, Tiezzi A, Pisani T, Munzi S, et al. (2009) Antiproliferative activity of lichen extracts on murine myeloma cells. Biologia (Bratisl) 64: 59–62.
- Koparal AT, Ulus G, Zeytinoğlu M, Tay T, Türk AÖ (2010) Angiogenesis inhibition by a lichen compound olivetoric acid. Phytother. Res 24: 754–758.
- Bessadóttir M, Skúladóttir EÁ, Gowan S, Eccles S, Ómarsdóttir S, et al. (2014) Effects of anti-proliferative lichen metabolite, protolichesterinic acid on fatty acid synthase, cell signalling and drug response in breast cancer cells. Phytomedicine 21: 1717–1724.
- Kosanić M, Ranković B, Stanojković T, Rančić A, Manojlović N (2014) Cladonia lichens and their major metabolites as possible natural antioxidant, antimicrobial and anticancer agents. LWT – Food Sci. Technol 59: 518–525.
- Ranković B, Kosanić M, Stanojković T (2014) Stereocaulon paschale lichen as antioxidant, antimicrobial and anticancer agent. Farmacia 62: 306–17.
- Grujičić D, Stošić I, Kosanić M, Stanojković T, Ranković B, et al. (2014) Evaluation of in vitro antioxidant, antimicrobial, genotoxic and anticancer activities of lichen Cetraria islandica. Cytotechnology 66: 803–813.
- Shrestha G, El‐Naggar AM, Clair LLS, O’Neill KL (2015) Anticancer Activities of Selected Species of North American Lichen Extracts. Phytother. Res 29: 100–107.
- Ari F, Ulukaya E, Oran S, Celikler S, Ozturk S, et al. (2015) Promising anticancer activity of a lichen, Parmelia sulcata Taylor, against breast cancer cell lines and genotoxic effect on human lymphocytes. Cytotechnology 67: 531–543.
- Yang Y, Park SY, Nguyen TT, Yu YH, Nguyen TV, et al. (2015) Lichen Secondary Metabolite, Physciosporin, Inhibits Lung Cancer Cell Motility. PLOS ONE 10: e0137889.
- Basile A, Rigano D, Loppi S, Di Santi A, Nebbioso A, et al. (2015) Antiproliferative, Antibacterial and Antifungal Activity of the Lichen Xanthoria parietina and Its Secondary Metabolite Parietin. Int. J. Mol. Sci 16: 7861–7875.
- Fernández-Moriano C, Divakar PK, Crespo A, Gómez-Serranillos MP (2015) Neuroprotective activity and cytotoxic potential of two Parmeliaceae lichens: Identification of active compounds. Phytomedicine 22: 847–855.
- Emsen B, Aslan A, Togar B, Turkez H (2016) In vitro antitumor activities of the lichen compounds olivetoric, physodic and psoromic acid in rat neuron and glioblastoma cells. Pharm. Biol 54: 1748–1762.
- Ristić S, Ranković B, Kosanić M, Stanojković T, Stamenković S, et al. (2016) Phytochemical study and antioxidant, antimicrobial and anticancer activities of Melanelia subaurifera and Melanelia fuliginosa lichens. J. Food Sci. Technol 53: 2804–2816.
- Yang Y, Nguyen TT, Jeong MH, Crişan F, Yu YH, et al. (2016) Inhibitory Activity of (+)-Usnic Acid against Non-Small Cell Lung Cancer Cell Motility. PLOS ONE 11: e0146575.
- Felczykowska A, Pastuszak-Skrzypczak A, Pawlik A, Bogucka K, Herman-Antosiewicz A, et al. (2017) Antibacterial and anticancer activities of acetone extracts from in vitro cultured lichen-forming fungi. BMC Complement. Altern. Med 17: 300.
- Suh SS, Kim T, Kim J, Hong JM, Nguyen T, et al. (2017) Anticancer Activity of Ramalin, a Secondary Metabolite from the Antarctic Lichen Ramalina terebrata, against Colorectal Cancer Cells. Molecules 22: 1361.
- Paluszczak J, Kleszcz R, Studzińska-Sroka E, Krajka-Kuźniak V (2018) Lichen-derived caperatic acid and physodic acid inhibit Wnt signaling in colorectal cancer cells. Mol. Cell Biochem 441: 109–124.
- Hong JM, Suh SS, Kim TK, Kim JE, Han SJ, et al. (2018) Anti-Cancer Activity of Lobaric Acid and Lobarstin Extracted from the Antarctic Lichen Stereocaulon alpnum. Molecules 23: 658.
- Nguyen TT, Chau TNQ, Van HM, Quoc TP, Phuoc QD, et al. (2019) A new hopane derivative from the lichen Dirinaria applanata. Nat. Prod. Res 0: 1–5.
- Nugraha AS, Pratoko DK, Damayanti YD, Lestari ND, Laksono TA, et al. (2019) Antibacterial and Anticancer Activities of Nine Lichens of Indonesian Java Island. J. Biol. Act. Prod. Nat 9: 39–46.
Table 3: UV screening compounds from lichens.
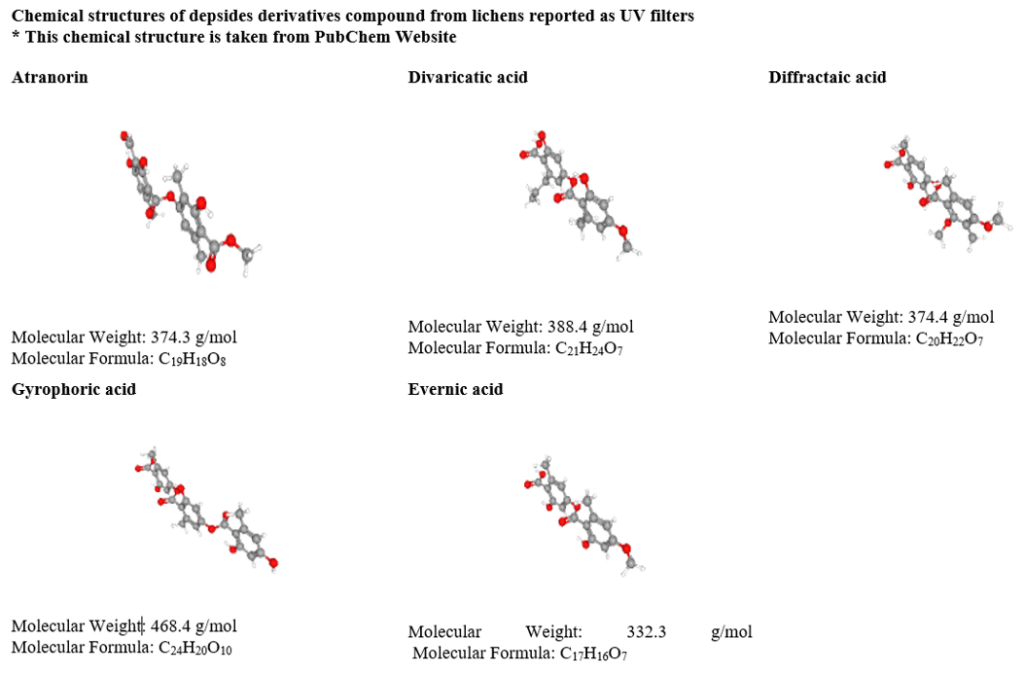
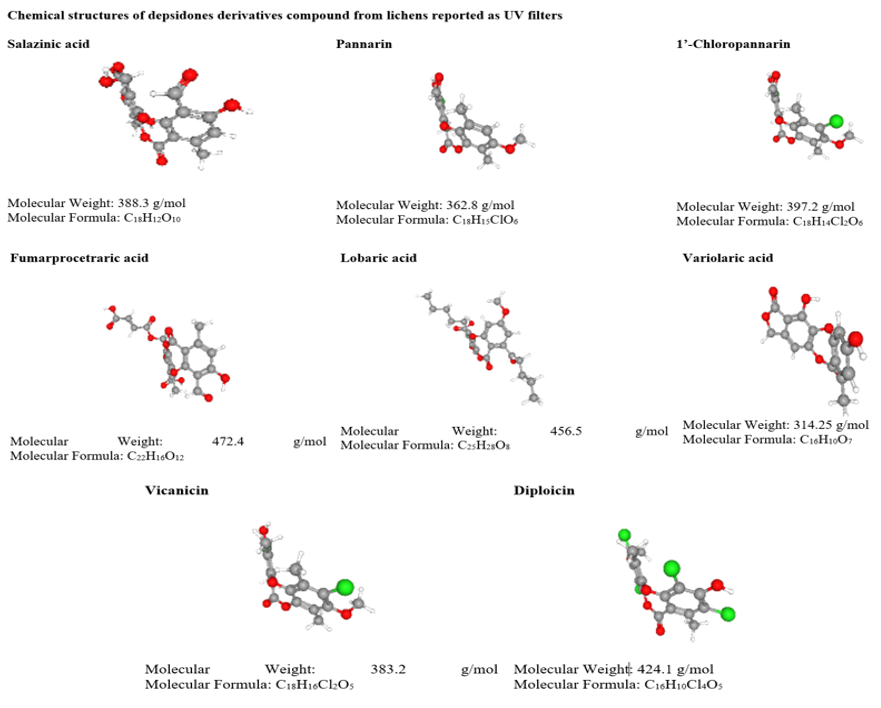
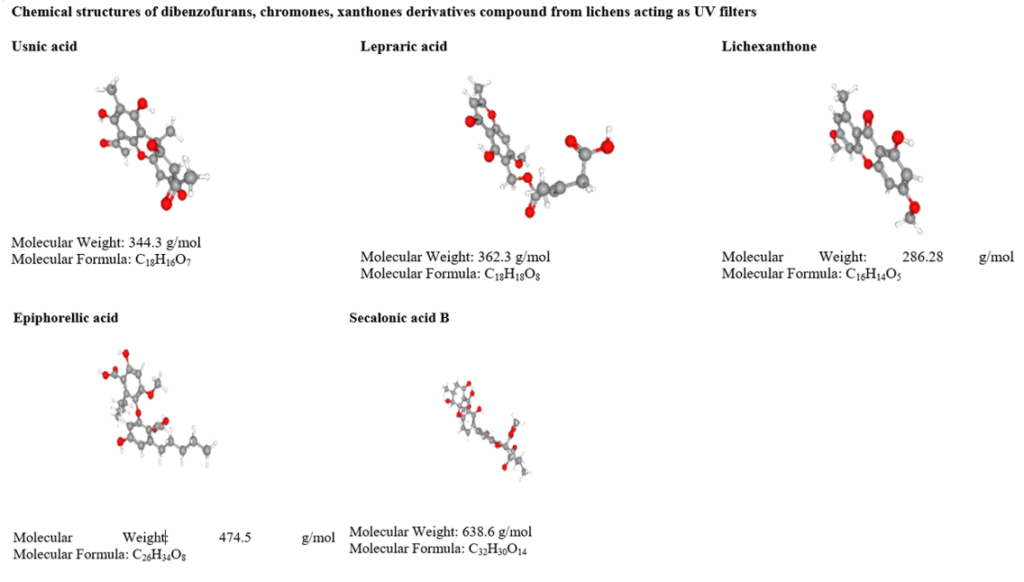
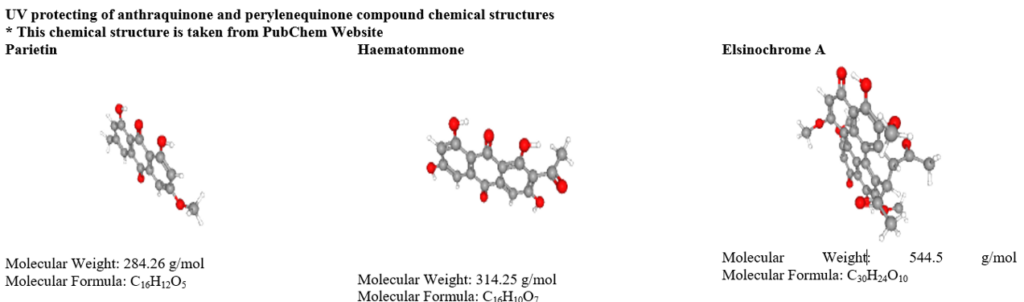
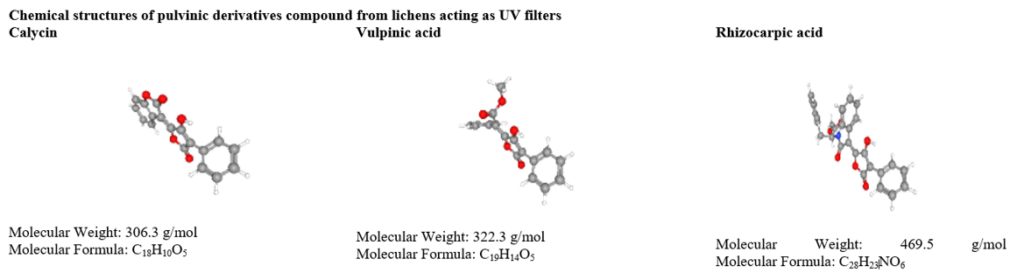
Table 4: Anticancer activity of lichen secondary metabolites.
|
Lichen compound/extracts |
Cell lines tested |
Major finding |
References |
|
Usnic acid derivatives |
L1210 Lewis lung carcinoma |
Seven out of eleven usnic acid derivatives completely inhibited L1210 cell growth at 1.4×10-7 mol/ml and it was found that the lipophilicity and β-triketone moiety of usnic acid were responsible for its cytotoxicity. |
[29] |
|
Lobaric acid Protolichesterinic acid |
T-47D & ZR-75-1 Breast cancer cell, K-563 Erythro-leukemia |
Significant apoptosis in cell lines were examined at 20 and 30 µg/ml concentrations of protolichestericnic acid and lobaric acid respectively. At higher concentrations proliferation, DNA synthesis and survival of fibroblasts in normal skin cells were unaffected. |
[103] |
|
Cladonia convoluata Cladonia rangiformis Evernia prunastri Parmelia perlata Parmelia caperata Ramalina cuspidata Usnea rubicunda extracts. |
3LL Murine Lewis lung carcinoma, L1210 Murine lymphocytic leukaemia, Human chronic myelogenous leukaemia, MCF7 Human breast adenocarcinoma, DU145 Human brain metastasis of prostate carcinoma, RCB-0461 Human glioblastoma, African green monkey kidney cell vero |
3 different solvent extracts such as diethyl ether, methanol and n- and hexane, of 8 species were evaluated for antiproliferative activity against seven cell lines with an IC50 value of <20µg/ml for one solvent extracts of each lichen species. Crude extracts of C. convoluta, C. rangiformis, P. caperata, P. glauca, and R. cuspidata were found to have high selectivity indices which suggests a vital role as anti-cancer agents. |
[162] |
|
Depsidones-Vicanicin, Pannarin, 1-chlotropannarin, Salazinic acid, Stictic acid, Variolaric acid, Psoromic acid, Fumarprotocetraric acid, Lobaric acid Depsides-Atranorin, Sphaerophorin, Divaricatic acid, diffractaic acid, gyrophoric acid Usnic acid |
Hepatocytes from rat |
Among 15 different lichen compounds analyzed, the cytotoxicity activity of usnic acid was higher with an IC50 value of 21 µg/ml after 20 h and lactic acid dehydrogenase was used for this purpose. Psoromic acid, stictic acid, and salazinic acid, showed concentration-dependent apoptosis of liver cell lines. The stictic acid showed higher apoptotic activity. |
[109] |
|
Sphaerophorin Pannarin Epiphorellic acid-1 |
DU 145 Human prostrate carcinoma,
|
All lichen compounds are nontoxic to normal prostatic human epithelial cells. On the basis of antiproliferative activity, the compounds such as Sphaerophorin, Pannarin and Epiphorellic acid-1 showed excellent cytotoxicity activity against DU-145 cells at a concentration of 6-50 µ mol/l results. Among these 3 compounds, Pannarin showed the maximum activity at a minimum inhibition concentration ranges between 12 and 25 µmol/I. The results showed that necrosis was induced at a higher concentration with the value greater than 50 µmol/I. The reason was due to the effect of lactic dehydrogenase induction. The examination of DNA fragmentation in DU 145 cells were higher at a dose of 12 and 25 µmol/I concentration. The effect of lichen compound to induce apotptosis was evident at this concentration that caused DNA damage. No such activities were seen at a concentration greater than 50 µmol/I. |
[49] |
|
(+) Usnic acid (-) Usnic acid |
V79 Lung fibroblast, A549 Human lung carcinoma |
The time and dose dependent cytotoxicity of usnic acid are involved to inhibit V79 and A549 cell lines. Cytotoxicity was less pronounced in V79 than A549. |
[47] |
|
Cetraria aculeata extract |
HeLa Human uterus carcinoma, A549 Human lung carcinoma, F2408 Rat embryonic fibroblasts, 5RP7 c-H-ras transformed rat embryonic fibroblast. |
The extract of Cetraria aculeata was found to be antiproliferative against A549 and HeLa with an IC50 value of 500 and 200 µg/ml respectively. Significant cytotoxic activity 5RP7 with IC50 values ranges between 80 and 280 µg/ml was found on F2408 cell line with the extract of Cetraria aculeata.. |
[93] |
|
Lethariella zahlbruckneri extract |
HT-29 Human colon cancer cell |
The crude methanolic and acetone extracts of L. zahlbruckneri reduced cell proliferation in both a dose and time dependent manner while an methanolic extract displayed lower cytotoxicity than the acetone extract. The acetone extract induced apoptosis by increasing cell proliferation in the sub-G1 phase, as well as the observation of nuclear condensation and apoptotic bodies while such results were not observed with methanolic extract. The induction of apoptosis by the acetone extract was mitochondria mediated in a caspase dependent and caspase independent mechanism. It was found that there is decreased level of the Bcl-2 protein and increased level of Bax. |
[41]
|
|
16-O– Acetyl-leucotylic acid Leucotylic acid |
HL-60 |
16-O– Acetyl-leucotylic acid was found to possess cytotoxic activity against HL-60 cell line with an EC50 value of 21µM. But the leucotylic acid, showed higher EC50 value. The higher cytotoxic activity of these two compounds were due to modification of its structure. Lesser cytotoxic activity was observed in Leucotylic acid than 16-O– Acetyl-leucotylic acid.
|
[163] |
|
Evernia prunastri extract Xanthoria parietina extract |
P3X63 Murine myeloma |
Significant cytotoxic effect was observed in a crude solvent extracts of Xanthoria parietina in a dose dependent manner while similar activities were not seen with Evernia prunastri. The considerable activity of X.parietina extract could be due to its higher antioxidant content viz., superoxide dismutase and peroxidases |
[163] |
|
Lecanoric acid Orsellinate derivatives |
MCF-7 Breast carcinoma, 786-0 Kidney carcinoma, HEP-2 Larynx carcinoma, B16-F-10 Murine melanoma cell |
Increase in antiproliferative activity of lecanoric acid was found in its modified structures. The compound is a derivative of orsellinates. The IC50 Value of orsellinate was found to be lesser than than lecanoric acid, The IC50 values of n-Butyl orsellinate showed its range between 7.2 and 14.0 µg/ml. The orsellinate activity was found higher corresponding to its lengthy chain. The results of lipophilicity was found to be higher in lengthy chain of orsellinate . |
[44] |
|
Olivetoric acid |
Rat adipose tissue |
Dose dependent anti-angiogenic activities was investigated with Olivetoric acid and it showed strong antiproliferative activity and degenerated endothelial tube development in adipose tissue. Accordingly, Olivetoric acid triggers dose dependent inhibition of actin stress fibres was examined. |
[164] |
|
Retigeric acid A Retigeric acid B |
PC-3, DU 145, Human Pca LNCaP, KB Human epidermoid cancer, 3-AO Human ovarian cancer, RWPEI Human benign prostate epithelial |
Lichen metabolites such as retigeric acid A (RA) and retigeric acid B (RB) displayed antiproliferative activity at a concentration more than 100µm and RA was less effective than RB. Structural relationship of RA and RB is -COOH substitution in RB. Lichen acids of RB were found to induce a concentration dependent accumulation of cells on PC-3 cell lines during the S phase of cell cycle followed by decrease in cyclin B, and increase in cyclin E and cyclin A. The results showed that caspase independent and dependent pathways activated apoptosis. |
[40] |
|
Usnic acid Atranorin Gyrophoric acid Parietin
|
A2780 Human ovarian carcinoma HT-29 Human colon adenocarcinoma |
Usnic acid and atranorin treated HT-29 cells cell lines showed caspase-3 activation and decreased mictochondrial membrane potential. Both test substances caused an externalization of phosphatidylserine in cell lines. Significant cell deaths in A2780 and HT-29 were observed. This may be due to mitochondrial pathway. |
[100] |
|
Diffractaic acid Vicanicin Lobaric acid Variolaric acid Protolichesterinic acid Usnic acid |
MCF-7 Human breast adenocarcinoma, HCT-116 Human colon adenocarcinoma, HeLa Human cervix adenocarcinoma |
Lichen acids displayed different antiproliferative action with higher cytotoxicity in HCT-116 but less activity in MCF-7. Of six compounds examined, vicanicin did not show any activity, while usnic acid and diffractaic acid were active against all 3 cancer cell lines. Protolichesterinic acid showed apoptosis induction after 72 h of treatment and a significant (3.27%) increase of caspase-3 activity was observed |
[45] |
|
Protolichesterinic acid |
SK-BR-3, T-47D Human breast cancer cell lines |
Ptotolichesterinic acid displayed significant cell deaths in cell lines of SK-BR-3, T-47D cell lines were observed with the IC50 values of 10.8, 11.7 µM. |
[165] |
|
Atranorin Fumarprotocetraric acid |
Fem-x Human melanoma and LS174 Human colon carcinoma cell line |
Fumar protocetraric acid and atranorin were evaluated for cytotoxicity against FemX and LS174 cells. Atranorin was most active with an IC50 value was 28.27, 20.88 µg/ml. The cytofluorimetric analysis was carried out for this purpose using propidium iodide labelled DNA. |
[166] |
|
Stereocaulon paschale extract |
Fem-x Human melanoma and LS174 Human colon carcinoma cell line |
Extract has strong anticancer activity against LS174 Human colon carcinoma and Fem-x Human melanoma cell line cell lines with the IC50 values of 40.22 and 23.52 µg/ml respectively. |
[167] |
|
Cetraria islandica extract |
Fem-x Human melanoma and LS174 Human colon carcinoma cell line |
Methanolic extract of C. islandica showed cytotoxic effects on LS174 and FemX cell lines with the IC50 values of 33.74 and 22.68 µg/ml. |
[168] |
|
Xanthoparmelia chlorochroa Tuckermannopsis ciliaris and 15 Lichen species extracts |
Burkitt’s lymphoma cells |
The extract of 14 species showed cytotoxicity activity against lymphoma cells. Both test substances of X. chlorochroa and T. ciliaris caused a significant decrease in cell proliferation and p53 upregulation. The extract of T. ciliaris upregulated TK1 expression but the extract of X. chlorochroa did not show TK1 gene expression. |
[169] |
|
Parmelia sulcata extract |
MCF-7 and MDA-MB-231 Breast cancer cell line |
P. sulcata extract showed significant anticancer activity against MDA-MB-231 and MCF-7 cell lines with the IC50 values of 16.5 µg/ml and 39.1 µg/ml respectively. The extract activiated apoptosis probably through the caspase independent pathway in these cells or involvement of caspase-3 mechanism. |
[170] |
|
Physciosporin compound and Pseudocyphellaria coriacea extract |
A549, H1650 and H1975 Human lung cancer cells |
The lichen metabolite physciosporin showed significant inhibitory activity against human lung cancer cells. Physciosporin treated cells showed both mRNA and protein levels of N-cadherin with significant decrease in the levels of epithelial-mesenchymal transition markers such as snail and twist. |
[171] |
|
Parietin compound and Xanthoria parietina extract |
MCF-7 and MDA-MB231 breast cancer cells |
The extract of Xanthoria parietina showed antiproliferation activity and induced apoptosis. Further investigation on the effects of parietin on MCF-7 and MDA-MB231 breast cancer cells was accompanied by alteration on expression of regulating genes such as P16, p27, cyclin D1 and cyclin A. |
[172] |
|
Cetraria islandica, Vulpicida canadensis extracts |
HepG2 Hepatocellular carcinoma, MCF-7 breast adenocarcinoma |
The lichen extracts of Cetraria islandica, and Vulpicida canadensis exhibited potent anticancer activity against MCF-7, HepG2 cell lines with the IC50 Values of and 19.51, 148.42 µg/ml.and 181.05, 58.02 µg/ml respectively. |
[173] |
|
Olivetoric acid, Physodic acid and Psoromic acid |
GBM and U87 MG Human brain cancer cells, PRCC Primary rat cerebral cortex cells |
Antiproliferative analysis using MTT assay showed that the metabolites exhibited higher susceptibility in cancer cells at a concentration of 40 mg/ml. Olivetoric acid showed strong cytotoxic effects for U87 MG and PRCC cells. Physodic acid showed less effective cytotoxic activity for both cells. |
[174] |
|
Lecanoric acid and 2’-O-methyl anziaic acid compounds, Melanelia subaurifera, Melanelia fuliginosa extracts |
Hela Human epithelial carcinoma, A549 Human lung cancer, LS174 Human colon carcinoma |
M. subaurifera extract was found to be cytotoxic against Hela, LS174, A549 cells with the IC50 values were 31.25, 9.88, 31.64 µg/ml repsectively. The lichen compounds lecanoric acid and 2’-O-methyl anziaic acid displayed less activity. |
[175] |
|
Alectoria samentosa, Flavocetraria nivalis, Alectoria ochroleuca, Usnea florida, Usnic acid compound |
A549 Lung cancer cell |
Usnic acid caused significant reduction in H1650 and H1975 cell lines at a concentration of 5 µM. The extract of Flavocetraria nivalis showed 60% cytotoxic effect. |
[176] |
|
Xanthoria parietina, Caloplaca pusilla and Protoparmeliopsis muralis lichen mycobiont extracts |
PC-3 Human prostate cancer, MCF-7 Human breast adenocarcinoma, HeLa Human cervix adenocarcinoma |
The extract of Caloplaca pusilla increased the cytotoxicity in HeLa, MCF-7, PC-3 with IC50 Values of 6.57, 7.29, 7.96 µg/ml-1 respectively. The mycobiont extract of Protoparmeliopsis muralis mycobiont did not display any anticancer activity. |
[177] |
|
Ramalin |
HCT116 Human colorectal cancer cell line |
Ramalina extract showed significant cell deaths and observed apoptosis in HCT116. The extract of Ramalina caused a significant increase in the expression of its downstream gene CDKN1A and TP53 while reduction in the expression of and CCNB1 and CDK1 on the basis of concentration dependent manner. |
[178] |
|
Atranorin Gyrophoric acid Physodic acid |
A375 Human melanoma cell line |
The test substance caused a significant antitumour activity at a concentratopn of 12.5-50µM. Atranorin and gyrophoric acid displayed lower less activity. Physodic acid activity has been found to increase Hsp70 expression a likely consequence of its interaction with regulatory elements and induces apoptosis. |
[111] |
|
Atranorin, Lecanoric acid, Caperatic acid, Physodic acid, Squamatic acid and Salazinic acid |
HCT116, DLD-1 Colorectal carcinoma and HaCaT cells |
HCT116 cells were sensitive to the Lecanoric and Caperatic acid and decreased Auxin 2 expression. Physodic acid effectively reduced Axin2 expression in HCT116 cells and less in DLD-1 cells. |
[179] |
|
Lobaric acid, Lobarstin |
HeLa Human cervix adenocarcinoma and HCT116 Colon carcinoma |
Extracts of lichen compounds such as lobaric acid and lobarstin exhibited cytotoxic activity in HCT116 and HeLa cancer cells and induce apoptosis at the G2/M phase. |
[180] |
|
Parmotrema tinctorum, Usnea rubrotincta, Usnea nipparensis, Lobaria pulmonaria and 10 lichen extracts.
|
MCF-7 Human breast adenocarcinoma, MO-91 Acute myelogenous leukemia cells |
The methanolic extract of Usnea nipparensis showed strong antiproliferative activity against MCF-7 cell lines with the IC50 values of 34.27 mg/ml-1. All the other tested lichen extracts showed different cytotoxic activity against MO-91 cell with the IC 50 value ranges between 10.50 and 50 mg/ml-1. |
[181] |
|
Parmelia tinctorum, Parmelia cetrata, Candelaria fibrosa, Clodonia scabriuscula, Teloschistes flavicans and 4 lichen extracts |
Vero normal cell, MCF-7 breast cancer, HeLa cervical cancer cell, WiDr colon cancer cells |
The methanolic extract of Cladonia scabriuscula was effective against MCF-7 and HeLa IC50 Values of 324 and 474 µg/ml respectively. |
[182] |


