Evaluation of nutritional profile and anti-oxidant activity of Meretrix meretrix Asiatic hard clam along the Parangipettai coast of Tamilnadu Kalimuthu Ramamoorthy1*, Govindarajan Sankar2, Somasekharan Nair Jeyapriya2
Kalimuthu Ramamoorthy1*, Govindarajan Sankar2, Somasekharan Nair Jeyapriya2
1Faculty of Marine Sciences, CAS in Marine Biology, Annamalai University, Parangipettai, Cuddalore – 608502, Tamilnadu, India
2Kalaignar Karunanithi Government Arts College for Women (A), Pudukkottai-622001, Tamilnadu, India
*Correspondence to: Kalimuthu Ramamoorthy
Citation: Ramamoorthy K, Sankar G, Jeyapriya SN (2023) Evaluation of nutritional profile and anti-oxidant activity of Meretrix meretrix Asiatic hard clam along the Parangipettai coast of Tamilnadu. Sci Academique 4(1): 15-35
Received: 29 April, 2023; Accepted: 11 May 2023; Publication: 16 May 2023
Abstract
The current study was conducted to determine the nutritional profile and antioxidant activity of the marine clam Meretrix meretrix. The proximate composition, vitamins, and mineral content of the sample were determined by standard biochemical methods. Amino acid and fatty acid profile were analyzed by High performance liquid chromatography (HPLC) and Gas Chromatography (GC) methods. The results showed that M. meretrix possessed prominent protein content with rich in essential amino acids (60.96%). Subsequently, the bioactive materials from M. meretrix using Acetone & n-hexane (1:3) extract ((AHMME) exhibited potential activity against the total antioxidant activity, superoxide radical scavenging assay, hydroxyl radical scavenging assay, DPH (1, 1- diphenyl – 2 – picrylhydrazyl hydrate) radical scavenging activity, reducing power assay, and ferrous ion chelating assay. Thus, the results of the present study suggest that the M. meretrix can be used as a potential food source for providing the cheapest animal protein and powerful natural antioxidants.
Scavenging effect (%) = {1-Sample (560nm)/Control (560nm) ×100}
Hydroxyl radical scavenging assay. Hydroxyl radical scavenging assay was calculated by the standard method (Aruoma and Halliwell, 1988). The reaction mixture containing AHMME and standard ascorbic acid were taken in the concentration of 25, 50, 75 and 100 µM respectively. In this concentration was incubated with deoxyribose (3.75 nm), H2O2 (1 mM, pH 7.4) at 37 oC. The reaction was terminated by adding TBA (1%, w/v) and TCA (2%, w/w) and then test tubes were incubated at 100ºC for 20 min. The contents were cooled and absorbance (OD) of the mixture was measured at 535 nm against reagent blank. Absorbance of reaction mixture was directly proportional to the decreasing rate of oxidation of deoxyribose.
DPH (1, 1- Diphenyl – 2 – Picrylhydrazyl Hydrate) radical scavenging activity. DPH free radical scavenging ability of molluscan extract was assessed (Shimada et al., 1992). AHMME and standard ascorbic acid were taken in the concentration of 25, 50, 75 and 100 µM respectively. In this concentration was mixed with methanol solution containing DPH radicals, resulting in a final concentration of 10 mM/1DPH. The mixture was shaken vigorously and left to stand for 30 minutes in the dark and the absorbance was measured at 517 nm against blank.
Reducing power assay. Reducing power of the AHMME was quantified by the method described earlier (Yen and Chen, 1995). The reaction mixture containing different concentrations of AHMME and standard ascorbic acid were taken in the concentration of 25, 50, 75 and 100 µM respectively. In this concentration, phosphate saline buffer (0.2 M, pH 6.6) was incubated at 50 oC for 20 min with potassium ferric cyanide (1%, w/v) at 50 oC. The reaction was terminated by adding TCA solution (10%, w/v) and the absorbance was measured at 700 nm. The increased absorbance of the reaction mixture indicated increased reducing power.
Ferrous-ion-chelating assay. The ferrous ion chelating potential of AHMME extract was investigated according to the method (Decker and Welch, 1990). The Fe2+ chelating ability of extract was monitored by measuring ferrozine complex at 562 nm. The reaction mixture containing AHMME and standard EDTA were taken in the concentration of 25, 50, 75 and 100µM respectively. In this concentration, FeCl2 (2 mM) and ferrozine (5 nM) was adjusted to a total volume of 0.8 ml with water, shaken well and incubated for 10 min. The absorbance of the mixture was measured at 562 nm against blank. EDTA was used as positive control and the ability of protein to chelate ferrous ion was calculated by following formula,
Chelating effect (%) = {1-Sample (562nm)/ Control (562nm)×Control) 562nm)}×100.
Statistical analysis. The experimental results were performed in triplicate. The data were recorded as Mean ± SD and analyzed by SPSS and followed by one way ANOVA. The difference was considered to be statistically significant at P<0.05 level.
Results
Proximate composition. In this present study the protein, carbohydrate, fat, ash and moisture content of M. meretrix were found to be 27.24 %, 11.95 %, 1.18 %, 4.92 % and 6.13 % respectively. Among the proximate compositions, the protein content was the predominant one (Figure 1).
Essential amino acids. In this study, a total number of 10 essential amino acids were recorded. Among the amino acids composition, Lysine 13.87%, Histidine 8.96%, and Methionine 9.13% were noted at higher concentrations and the remaining amino acids were observed in trace quantities (Figure 2).
Non-essential amino acids. Totally, nine non-essential amino acids were recorded from clam tissue samples. Among the non-essential amino acids, Alanine 5.63%, Aspartic acid 5.12%, Asparagine 3.83%, Tyrosine 3.59% and Proline 3.25% were noticed as predominant non-essential amino acids (Figure 3). The fatty acid assessment of M. meretrix showed six different groups of fatty acids. It includes two saturated fatty acids (SFA), one monounsaturated fatty acids (MUFA) and three polyunsaturated fatty acids (PUFA). Among the SFAs palmitic acid (C16:0) was recorded with major percentage. The percentage availability of SFA, MUFA and PUFA content were found to be 38.2 %, 12.47 % and 37.45 % respectively (Figure 4).
Vitamin composition. The Vitamin compositions of the present investigation were represented in Figure 5. Totally seven vitamins were recorded, Among which Vitamin A (108.4 IU) was observed at highest level followed by Vitamin C and D, with 23.98 mg/g, 13.82 IU,2.08 µg/g. Vitamin B12, E, K and B6 were recorded in meager quantities of1.98, 1.14, 0.59 and 0.31 respectively.
Mineral composition. Themineral composition analysis of study species showed higher and moderates levels of various minerals. The higher concentration of Calcium 314.23 mg/g, Sodium 88.32mg/g, and Magnesium 60.21 mg/g were recorded, while the Potassium, Zinc, Iron and Coper were recorded in inadequate quantities (Figure 6).
Total antioxidant activity. AHMME showed maximum total antioxidant activity (%) of 72.26±0.30 at 100µgml-1 and minimum activity was observed in 25 µg/ml-1. Similarly the standard ascorbic acid showed maximum 71.7±0.81 at 100 μM and minimum activity at 25 μM/ml-1 was observed. The study of AHMME delivered significant result compared to standard ascorbic acid and the total antioxidant activity was found directly proportional manner (Figure 7).
Superoxide radical scavenging activity. AHMME showed significant superoxide anion radical scavenging activity when compared with the standard ascorbic acid (70.5±0.45). The maximum (71±1.13 at 100 µgml-1) scavenging activity of AHMME was recorded and 100 µgml-1 minimum was 39.26±0.25 at 25 µgml-1 concentration (Figure 8).
Hydroxyl radical scavenging assay. The hydroxyl radical scavenging assay of molluscan extract showed maximum 50.53±0.50 µg ml-1 at the highest concentration of 100 µg ml-1 and minimum was 12.53±0.50 µg/ml at 25 µgml-1. The result of present study of AHMME extract was almost similar to standard ascorbic acid (60.15±0.25 at 100 µgml-1) (Figure 9).
DPH (1, 1- diphenyl – 2 – picrylhydrazyl hydrate) radical scavenging activity. The maximum scavenging ability (%) of 35.36±0.40 and 60.4±0.40 at 100 µg/ml were recorded in AHMME and standard ascorbic acid respectively. The minimum scavenging ability (%) of 10.82±0.02 was observed in the molluscan extracts at the concentration of 25 µg/ml. As compared to standard, the molluscan extract showed acceptable scavenging activity (Figure 10).
Reducing Power assay. It relies on the concentration of the extract and the standard reference drug. As the concentration increases reducing power ability of the test sample also increases. The reducing power (%) of bivalve extract AHMME showed highest activity 0.83±0.03 at 100 µg ml-1 concentration and lowest reducing power was recorded 0.12±0.005 at 25 µgml-1 concentration respectively. The standard ascorbic acid used showed maximum reducing activity 1.76±0.02± at 100 µgml-1 concentrations (Figure 11).
Ferrous Ion chelating assay. The ferrous ion-chelating assays of AHMME was found to be concentration dependent and displayed as in Figure. 12. The chelating effect (%) of AHMME showed highest activity at 100µgml-1 58.3±0.30 and lowest at 25 µgml-1 7.23±0.25 concentration. The standard showed chelating activity (%) of 83.33±0.30 at 100 µgml-1 concentration.
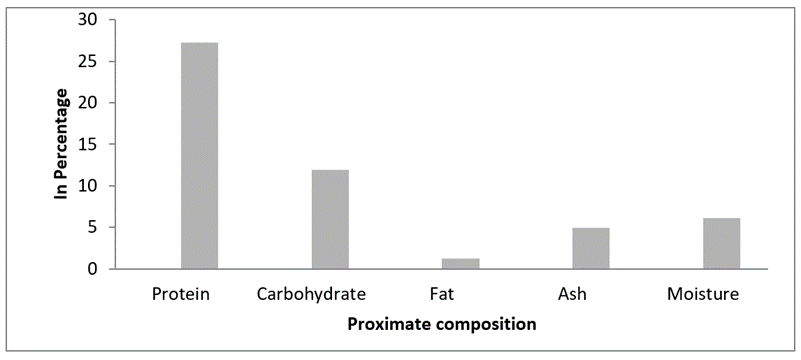
Figure 1: Proximate composition of M. meretrix.
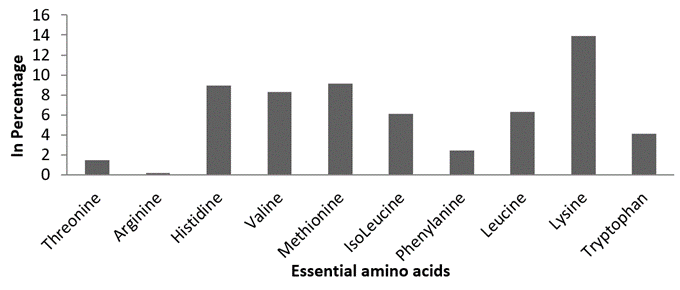
Figure 2: Estimated essential amino acid composition of M. meretrix.
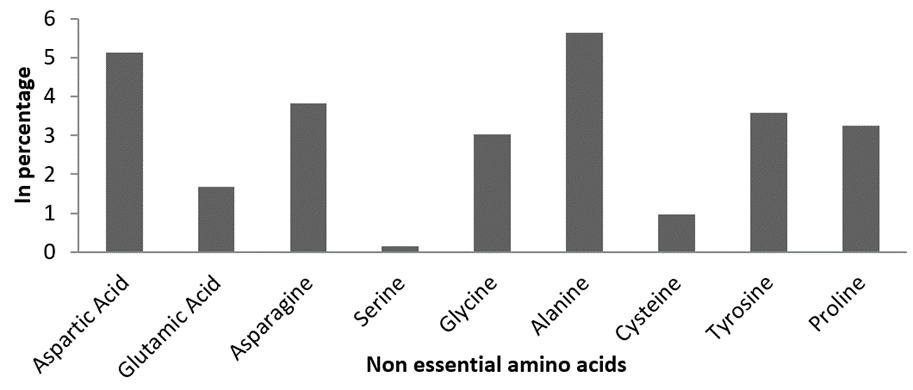
Figure 3: Estimated non-essential amino acid composition of M. meretrix.
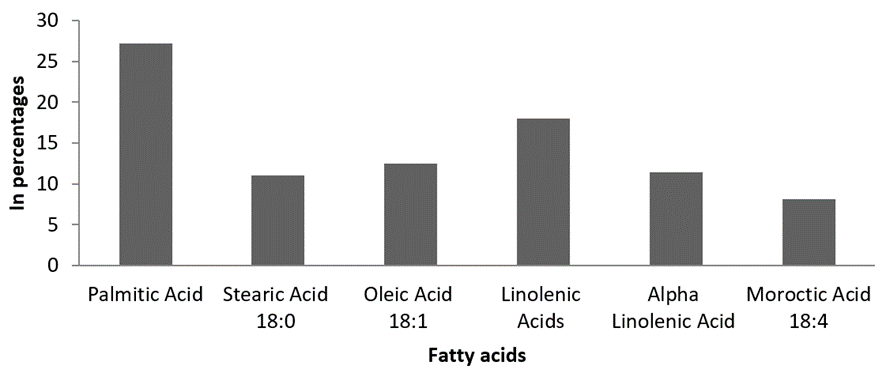
Figure 4: Estimated fatty acids composition of M. meretrix.
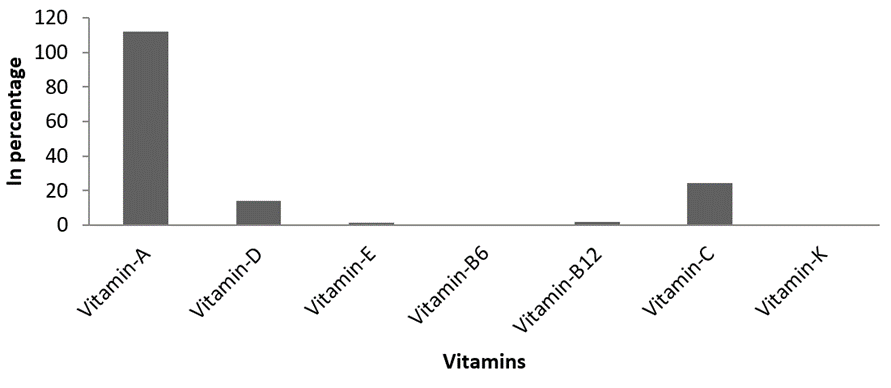
Figure 5: Estimated vitamin content of M. meretrix.
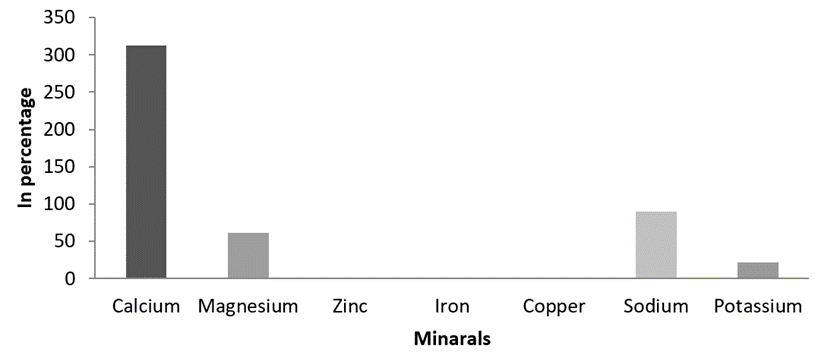
Figure 6: Estimated mineral content of M. meretrix.
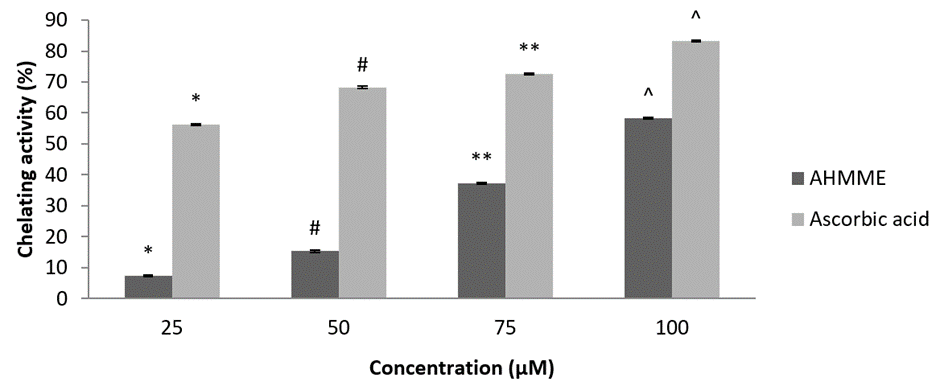
Figure 7: Total antioxidant activity of AHMME.
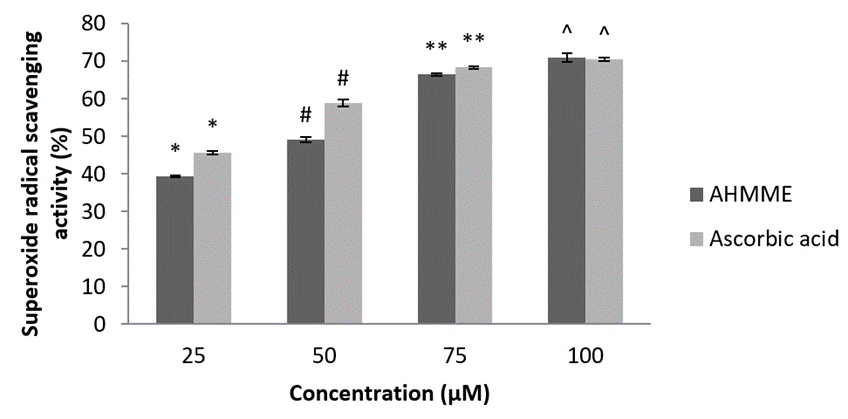
Figure 8: Superoxide radical scavenging activities of AHMME.
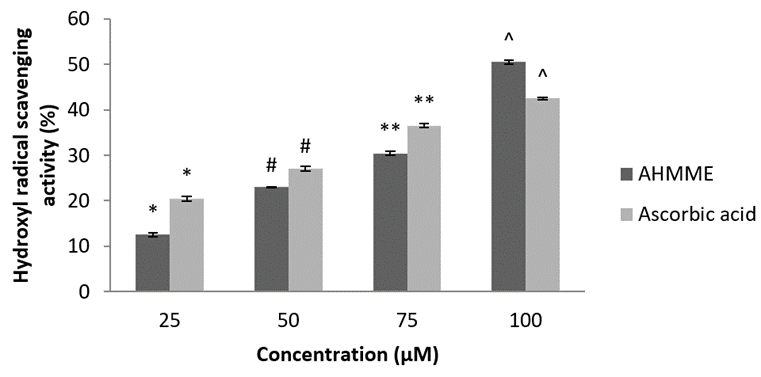
Figure 9: Hydroxyl radical scavenging activities of molluscan extract of AHMME.
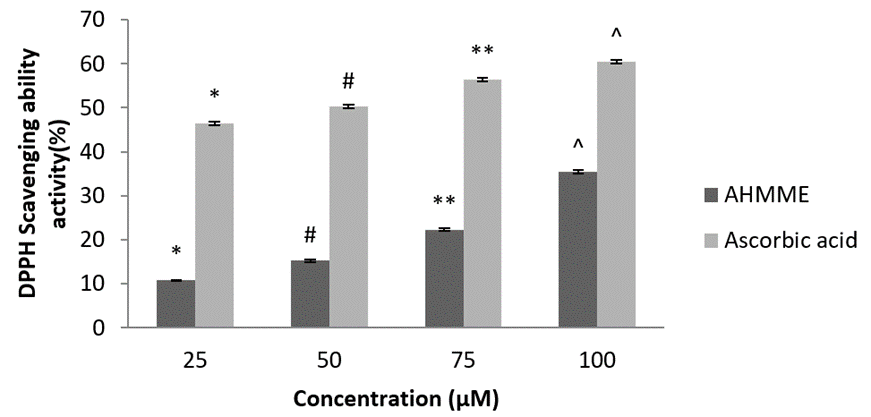
Figure 10: Scavenging ability of the AHMME on DPH.
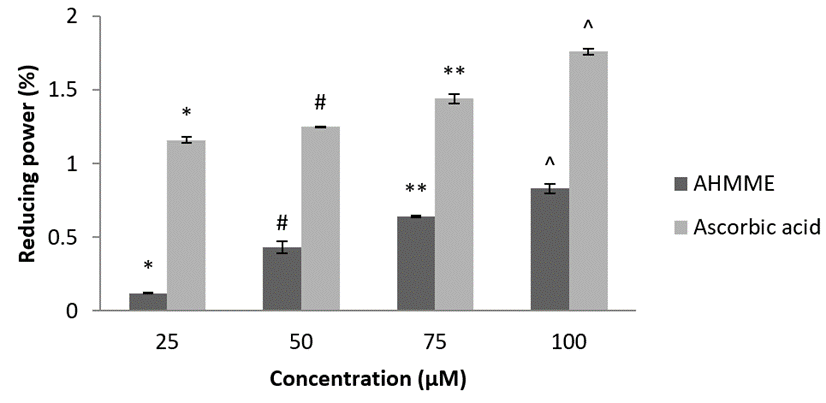
Figure 11: Reducing power of bivalve extract AHMME.
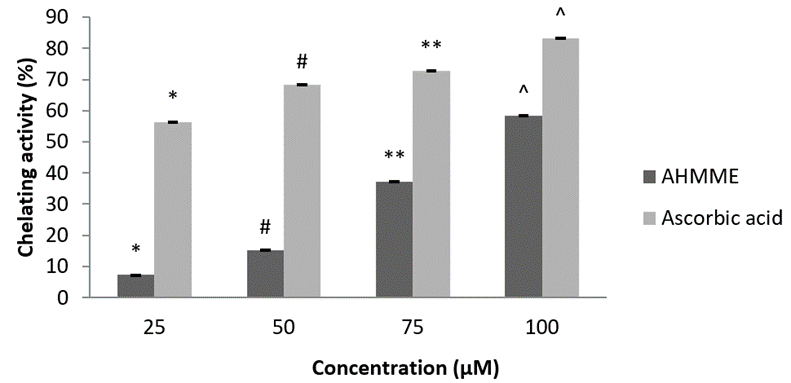
Figure 12: Ferrous ion chelating ability of AHMME.
Discussion
Marine foods are the most essential food source with low cost and higher nutritional values. Marine bioactive natural compounds have drawn the attention of many researchers in recent years because of their pharmacological values. Protein is an indispensable for the nourishment of life and exists in major quantity of all nutrients as a component of human body (Okuzum and Fujii, 2000). Present investigation revealed that the protein content is prominent when it is compared with carbohydrate and lipids contents on M. meretrix. Therefore, the results suggest that the M. meretrix can be considered as another potential food source for providing cheapest animal protein. Some researchers have reported 11.9% of protein has been observed in surf clam Mactra violacea (Laxmilatha, 2009). Beside clams the extract of Gonggong sea snails was found to be an excellent source of antimicrobial peptide (Viruly et al., 2022). Moreover, the protein level of Bursa spinosa was varied from 18.71 to 29.81% at Parangipettai coast (Subhapradha et al., 2013). The protein 23.51% was on marine bivalve Donax incarnates at Cuddalore coast (Periyasamy et al., 2013).
In the present study M. meretrix showed carbohydrate, fat, ash and moisture content at the range of 11.95%, 1.18%, 4.92% and 6.13% respectively. Carbohydrates comprise of sugars, starches and fiber, and acts as a major source of energy for animals. The carbohydrate level at Parangipettai coast on Catfish, Arius maculatus, Plotosus lineatus and puffer fish of Lagocephalus inermis and Lagocephalus lunaris was analyzed (Manikandarajan et al., 2014) and the carbohydrate levels varied from 2.15 gm to 1.98 gm head and body part of the cat fish, 1.87 % and 1.96 % at whole body tissue of Puffer fish. Maximum carbohydrate level of 9.2 % and minimum level of 8.3 % was recorded on Babylonia spirata in the gonad and digestive glands (Shanmugam et al., 2006). When compared with fish, the molluscan species are very cheap and economically less valued species that can substantiate the fish nutritional supplements.
The lipids are highly well-organized source of energy, in that they contain more than twice the energy of carbohydrate and proteins. The lipid content 1.18% was noticed on an investigated clam sample. In male and female species of Rapana rapiformis, the lipid content was recorded to be 0.85-2.12% and 0.95- 2.96% respectively (Rajkumar, 1995). In Babylonia zeylanica and Pleuroploca trapezium species 10.38% and 1.97% of highest lipid content were noticed (Nirmal, 1995)
Protein values are obviously reflected in the essential amino acids concentrations. Present study found total of 19 amino acids in theM. meretrix. This study reveals that the clams were rich in essential amino acids (60.96%) than that of non-essential amino acids (27.24%). The result reveals that the meat of M. meretrix can be very good source for human diet due to an elevated level of quality protein, as well as balanced essential amino acids. The amino acid concentrations on different marine molluscs in Southeast Coast of India, was observed (Babu et al., 2010). They recorded that the total amino acid level in Perna viridis was 95.76%, Crassostrea madrasensis was 98.4% and in Meretrix casta was 65.17%. Strombus canarium was comprised of 80.97% of essential and 15.07% of non-essential amino acids, Bursa spinosa was comprised of 50.01% of essential amino acids and 46.79% of non-essential amino acids. In this context, current study clearly demonstrates that these marine mollusks might be a good potential source of amino acid.
Moreover, 28.13 % of palmitic acid and 18.74 % of Linolenic acid in D. incarnates from Cuddalore, Southeast coast of India was reported (Periyasamy et al., 1971). And Donax cuneatus contain saturated, mono and polyunsaturated fatty acids at the range of 35.28%, 12.71%, 11.72% respectively (Annaian et al., 2007). The results of the present study also showed that the marine animals are richest source of PUFA.
Molluscs contain a variety of minerals, vitamins, essential and non-essential amino acids and high quality protein (Periyasamy et al., 1971). The flesh of fish and shell fish are imperative sources of vitamin A (Pigott, 1990). In the present study, the Clam tissue showed the major source of vitamin A and vitamin C which constituted 108.4 IU and 23.98 mg/g. The vitamin levels on three different mollusc species green mussels (P. viridis), true oyster (C. madrasensis) and yellow clam species (M. casta) was studied (Ajaya Bhaskar, 2002). These species contain a significant level of Vitamins like B1 (0.11), B2 (031) and B6 (0.31). Shellfish enclosed in the present study, showed complete range of vitamins. The Vitamins content on Clam M. casta from Parangipettai and Cuddalore coast was estimated (Srilatha et al., 2014). According to the estimation, the composition is as follow: vitamin A (14.40IU, 8.200IU), vitamin D (200IU, 150IU), vitamin E (1.18 mg/g., 1.06 mg/g) and Vitamin K (0.62 mg/g, 0.18 mg/g). Minerals constitute important components of enzymes, hormones and enzyme activators (Khan et al., 2007). Mineral components such as sodium, potassium, magnesium, calcium, iron, phosphorus and sulphur are imperative nutrients for human diet (Erkan and Ozden, 2007).
The above discussion revealed that diminutive variations carotenoids of proximate contents were noticed when compared to the present data. The variation in concentration of clam nutrition might be due to the feeding behavior, environment, ecosystem and migratory event within the same region. In addition, the chemical composition of fish meat was found to vary with sex, season, size, age and geographical locality of catch (Zenebe et al., 1998).
Moreover, in the last three decades about 1000 research papers have been published on molluscs secondary metabolites (Avila, 2006), with a total of 729 compounds were reported from 199 species (Faulkner, 2009). Dietary antioxidants, for instance carotenoids aids in preventing several human diseases and potential antioxidants absorbs the excited energy from the singlet oxygen in to the carotenoid chain, that leads to degradation of carotenoid molecule thereby preventing the damages of other molecules and tissues (Buhl-Mortensen and Hoisaeter, 1993). They also prevent the production of free radicals induced by the poly-unsaturated fatty acids degradation. Moreover, Carotenoids prevents the peroxidation of membranous phospholipids and lipids (Naguib, 2000). Therefore, therapeutic interventions having antioxidants or free radicals scavenging activity may be useful against oxidative stress associated with various cardiovascular diseases including myocardial infarction (Rajadurai and Prince, 2007). AHMME was involved to evaluate the total antioxidant nature of it. It showed some promising results at high concentrations and the values were significantly similar when compared with the standard reference drug.
Superoxide anion is very harmful to cellular components. Numerous biological reactions generate superoxide anions which are highly toxic in nature. In PMS/NADH-NBT system, the superoxide anion derived from PMS/NADH coupling reaction reduces NBT. The decrease of absorption at 560 nm with antioxidants thus indicates the consumption of superoxide anion in the reaction mixture (Alagumanivasagam et al., 2012). In the present investigation, the superoxide radical scavenging activity of AHMME was comparable to standard reference compound ascorbic acid. In the above observation it is concluded that AHMME potent scavenging capacity of superoxide anion. Likewise the cuttlefish extract GAG scavenge the superoxide in a concentration dependent manner (Vino et al., 2012). Similarly, the superoxide anion radical scavenging ability of marine species were also dose dependent (Maximas et al., 2014).
Hydroxyl radical is one of the more potent reactive oxygen species in the biological system. It reacts with polyunsaturated fatty acid moieties of cell membrane phospholipids and cause damage to cell (Gutteridge et al., 1981). Hydroxyl radical causes severe damage in bio-molecules (Yan et al., 2005) and also plays a major role in lipid peroxidation. In the present analysis the hydroxyl radical scavenging effect of AHMME showed some promising hydroxyl radical scavenging activity and results are comparable to the standard ascorbic acid. Previously, chitosan from cuttlefish shows high levels of hydroxyl scavenging activity of 72.1% (Vino et al., 2012). Present results were lined with the previous findings AHMME reaction mixture scavenged the hydroxyl radicals from the sugar and prevented the reaction. It indicates that AHMME was proved as better hydroxyl radical scavenger.
DPH possess proton free radicals with characteristics absorbance which decrease significantly on exposure to proton radical scavengers. Further it is accepted that the DPH free radical scavenging by antioxidant is due to their hydrogen donating ability. Thus scavenging of DPH free radicals was directly affected by the amount of attractable hydrogen atoms in a protein molecule. The free radical scavenging ability using the DPH method was accessed to identify the antioxidant potential of marine species (Hong-Yu et al., 2010). In the present investigation AHMME reported strong DPH scavenging activity and it clearly indicates the hydrogen donating ability of Mollusca extract. Similar results were observed by several researchers on different marine species. The effect of antioxidant on DPH radical scavenging is thought to be due to their hydrogen donating ability (Conforti et al., 2009).
Reducing power assay has been used to evaluate the ability of natural antioxidants to donate electrons. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity (Dorman et al., 2003). The present data also showed excellent reducing capacity when compared to the standard drug. And the reducing power assay of Berberis tinctoria was also studied and it also showed dose dependent mannered activity which could be due to the free radical chain by denoting hydrogen atom (Sasikumar et al., 2012). Further suport that, the reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. In addition, the antioxidant activity of antioxidants has been attributed to various mechanisms, including the prevention of chain initiation, binding of transition metal ion catalysts, decomposition of peroxides, prevention of continued hydrogen abstraction, reductive capacity and radical scavenging (Yildirim et al., 2000).
Metal chelating capacity was significant, since it reduced the concentration of the catalyzing transition metal in lipid peroxidation. Previous report support that, chelating agents are effective as secondary metabolites, because they reduce redox potential, thereby stabilizing the oxidized form of metal ion (Gulcin et al., 2007). Present investigation was in agreement with the previous research on Meretrix casta extract, which also explained the antioxidant potential by carrying out DPH, Iron, chelating and reducing (Pachaiyapan et al., 2014). And Babylonia zeylanica produce remarkable antioxidant effect on iron chelating study by stabilizing the oxidized from of metal-ions (Velayutham et al., 2014).
Conclusion
The present study AHMME showed significant in-vitro free radical scavenging effects. And the results of the study strongly suggest that the free radical scavenging potential of AHMME noticed in dose dependent manner. Further the outputs of the nutritional profile showed that, M. meretrix have been recommended as a good source of food for human consumption in future. Further AHMME extract will be purified and characterized to evaluate their biological activity in future.
Acknowledgments: All the authors were thankful to CAS in Marine Biology, Annamalai University, Parangipettai, Tamilnadu, India and Kalaignar Karunanithi Government Arts College for Women (A), Pudukkottai, Tamilnadu, India for the necessary support to carry out this research study.
Conflict of Interest: Authors declare that no conflict of interest
References
- Ajaya Bhaskar D (2002) Nutritional evaluation of molluscan seafood. Ph.D. Thesis (Annamalai University, India,).
- Alagumanivasagam G, Pasupathy R, Kottaimuthu A, Manavalan RA (2012) Review on in-vitro antioxidant methods, Int J of Pharm Chem Sci, vol. 1, p. 662-74.
- Ananthi S, Raghavendran H.R.B, Sunil A.G, Gayathri V, et al, (2010) In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga), Food and chem toxi, vol. 48, p. 187-192.
- Annaian S, Chendur P, Subramanium S (2007) Some valuable fatty acids exposed from wedge clam Donax cuneatus (Linnaeus), Afri J of Biochem Res, vol. 1, p. 014-018.
- Aruoma O.I, Halliwell B (1988) The iron-binding and hydroxyl radical scavenging action of anti-inflammatory drugs, Xeno, vol. 18, p. 459-470.
- Association of Official Analytical Chemists [AOAC] (1990). Official Methods of Analysis.
- Avila C (2006) Molluscan natural products as biological models: chemical ecology, histology, and laboratory culture, Molluscs., p. 1-23.
- Babu A, Kesavan K, Annadurai D, Rajagopal S (2010) Bursa spinosa – A mesogastropod fit for human consumption, Adv J Fo Sci and Tech., vol. 2, p. 79-83.
- Barone R, De Santi C, Palma Esposito F, Tedesco P, Galati F, et al. (2014) Marine metagenomics, a valuable tool for enzymes and bioactive compounds discovery, Fron in Mar Sci, vol. 1, p. 38.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Analy bioche., vol. 72, p. 248-254.
- Buhl-Mortensen L, Hoisaeter T (1993) Mollusc fauna along an offshore-fjord gradient, Mari ecol prog ser, Oldendorf, vol. 97, p. 209-224.
- Conforti F, Menichini F, Formisano C, Rigano D, Senatore F, et al. (2009) Comparative chemical composition, free radical-scavenging and cytotoxic properties of essential oils of six Stachys species from different regions of the Mediterranean Area, Food Chem., vol. 116, p. 898-905.
- Decker EA, Welch B (1990) Role of ferritin as a lipid oxidation catalyst in muscle food, J Agric Food Chem, vol. 38, p. 674-677.
- Dorman H.J.D, Peltoketo A, Hiltunen R, Tikkanen MJ (2003), Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs, Food chem, vol. 83, p. 255-262.
- Dubois M, Gilles K.A, Hamilton J, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances, Analy chem., vol. 28, p. 350-356.
- Erkan N, Ozden O (2007) Proximate composition and mineral contents in aqua cultured sea bass (Dicentrarchus labrax), sea bream (Sparus aurata) analyzed by ICP-MS, Food chem, vol. 102, p. 721-725.
- Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition, Nutri, vol. 18, p. 872-879.
- Faulkner P (2009) Focused, intense and long-term: evidence for granular ark (Anadara granosa) exploitation from late Holocene shell mounds of Blue Mud Bay, northern Australia, J of Archae Sci., vol. 36, p. 821-834.
- Folch J, Lees M, Sloane Stanley G.H (1957) A simple method for the isolation and purification of total lipids from animal tissues, J biol Chem., vol. 226, p. 497-509.
- Geccelep H, Uzun Y, Tuncturk Y, Demirel K (2009) Determination of mineral contents of wild-grown edible mushrooms, Food Chem, vol. 113, p. 1033-1036.
- Gerwick W.H, Moore B.S (2012) Lessons from the past and charting the future of marine natural products drug discovery and chemical biology, Chem & bio, vol. 19, p. 85-98.
- Gorinstein S, Cvikrova M, Machackova I, Haruenkit R, Park Y.S, et al, (2004) Characterization of antioxidant compounds in Jaffa sweeties and white grapefruits, Food chem, vol. 84, p. 503-510.
- Gratzfeld-Huesgen A (1999) Sensitive and reliable amino acid analysis in protein hydrolysates using the Agilent 1100 Series HPLC, Technical Note by Agilent Technologies., p. 5968-5658E.
- Gulcin I, Elmastas M, Aboul‐Enein H.Y (2007) Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies, Phytotherapy Res: An Inter J Devoted to Pharm and Toxi Eval of Nat Prod Deriv., vol. 21, p. 354-361.
- Gutteridge J.M, Rowley D.A, Halliwell B (1981) Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of ‘free’ iron in biological systems by using bleomycin-dependent degradation of DNA, Biochem J, vol. 199, p. 263-265.
- Hong-Yu L, Bin W, Chun-Guang Y, You-le Q, Chuan-ling S (2010) Evaluation of antioxidant activities of five selected brown seaweeds from China, J of Med Pla Res, vol. 4, p. 2557-2565.
- Jayabal R, Kalyani M (1986) Age and growth of the estuarine clam Meretrix meretrix (L) inhabiting the Vellar estuary, Mahasagar, vol. 19, p. 141-146.
- Kamboj V.P (1999) in Ocean Science Trends and Future Directions, Ed. by B.L.K. Somayajulu. (Indian National Science Academy, New Delhi,), p. 197-227.
- Khan Z.I, Ashraf M, Ahmad K, Mustafa I, Danish M (2007) Evaluation of micro minerals composition of different grasses in relation to livestock requirements, Pak J Bot, vol. 39, p. 719-728.
- Laxmilatha P (2009) Proximate composition of the surf clam Mactra violacea (Gmelin 1791), Ind J Fish, vol. 56, p. 147-150.
- Lepage G, Roy C.C (1986) Direct trans-esterification of all classes of lipids in a one-step reaction, J lipid res., 1986, vol. 27, p. 114-120.
- Lingert H, Vellentinin K, Eriksson C.E (1979) Measurment of antioxidative effect in model system, J. Food Process Preserv, vol. 3, p. 87-103.
- Lodeiros C.J, Maeda-Martinez A, Freites L (2001) in Los Moluscos Pectínidos de Iberoamerica: Ciencia y Acuicultura, Ed. by A.N. Maeda-Martinez. (Limusa, Mexico,), p. 77-88.
- Manikandarajan T, Eswar A, Anbarasu R, Ramamoorthy K, Sankar G (2014) Proximate, amino acid, fatty acid, vitamins and mineral analysis of catfish, Arius maculatus and Plotosus lineatus from Parangipettai south east coast of India, IOSR J of Envi Sci, Toxiy & F Tech., vol. 8, p. 1-8.
- Maximas H.R, Sudha P.N, Sudhakar K, (2014) A study on the hepatoprotective activities of Methanol Extract of Spinacia oleracea (Linn.) to the Induced Hepatotoxicity in wistar rat models, Inter J Phar Res Health Sci., vol. 2, p. 287-301.
- Nagash Y.S, Nazeer R.A, Kumar N.S (2010) In vitro antioxidant activity of solvent extracts of mollusks (Loligo duvauceli and Donax strateus) from India, Wor J Fish Mar Sci., vol. 2, p. 240-245.
- Naguib Y.M (2000) Antioxidant activities of astaxanthin and related carotenoids, J of agri and food chem., vol. 48, p. 1150-1154.
- Nazar A.B.A, Adhila Beegam K.A, Skinner A, Dastidar D.G, Antony E.J, et al. (2022) Prophylaxis through Marine-derived bioactive compounds toward Neurodegenerative Disorders (Springer, Singapore,).
- Nirmal A (1995) Biochemical studies on prosobranchian gastropods Babylonia zeylonica (Neogastropods: Buccinidae: Fasciolariidae). M.Sc. Dissertation (Annamalai University, India,).
- Nishikimi M, Rao N.A, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen, Bioc and biop res com, vol. 46, p. 849-854.
- Okuzumi M, Fujii T (2000) Nutritional and functional properties of squid and cuttlefish, National Cooperative Association of Squid Processors.
- Pachaiyapan A, Muthuvel A, Sadhasivam G, Sankar V.J.V, Sridhar N, et al. (2014) In vitro antioxidant activity of different gastropods, bivalves and echinoderm by solvent extraction method, Inter J Pharma Sci and Res., vol. 5, p. 2539.
- Periyasamy N, Murugan S, Bharadhirajan P (2014) Biochemical composition of marine bivalve Donax incarnatus (Gmelin, 1791) from Cuddalore Southeast coast of India, Inter J adv in phar bio and chem., vol. 3, p. 575-582.
- Periyasamy N, Murugan S, Bharadhirajan P (2013) Isolation and characterization of anticoagulant compound from marine mollusc Donax faba (Gmelin, 1791) from Thazhanguda, Southeast Coast of India, Afr J Biot, vol. 12, p. 5968-5974.
- Pigott GM (1990) in Seafoods: Effects of technology on nutrition, Ed. by Pigott and Tucker. (Marcel Dekker, Inc, New York,), p. 294-314.
- Rajadurai M, Prince P.S.M (2007) Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats, Toxi., vol. 230, p. 178-188.
- Rajkumar T (1995) Studies on biology of Rapana rapiformis (Born) (Mollusca: Gastropoda: Rapanidae) from Parangipettai. Ph.D. Thesis (Annamalai University, India, ).
- Sadasivam S, Manickam A (1996) Peroxidase, Methods in Biochemistry. New Age International: New Delhi, p. 108-110.
- Sasikumar J.M, Maheshu V, Smilin A.G, Gincy MM, Joji C (2012) Antioxidant and antihemolytic activities of common Nilgiri barberry (Berberis tinctoria Lesch.) from south India, Inter Food Res Jou., vol. 19, p. 1601-1607.
- Shanmugam A, Bhuvaneswari T, Arumugam M, Nazeer R.A, Sambasivam S (2006) Tissue chemistry of Babylonia spirata (Linnaeus), Ind J Fish, vol. 53, p. 33-39.
- Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion, J of Agri chem., vol. 40, p. 945-948.
- Srilatha G, Eswar A, Manikandarajan T, Ramamoorthy K, Sankar G, et al. (2014) Recipes from yellow clam Meretrix casta (Chemnitz), Inter Let of Nat Sci., vol. 19, p. 25-33.
- Subhapradha N, Ramasamy P, Sudharsan S, Seedevi P, Moovendhan M, et al. (2013) Antioxidant potential of crude methanolic extract from whole body tissue of Bursa spinosa (SCHUMACHER, 1817), In Proceedings of the National conference-USSE., p. 163-167.
- Sugesh S, Mayavu P (2013) Antimicrobial activities of two edible bivalves M. meretrix and M. casta, Pak J. Bio Sci, vol. 16, p. 38-43.
- Velayutham S, Sivaprakasam RM, Williams S.F, Samuthirapandian R (2014) Potential activity of in vitro antioxidant on methanolic extract of Babylonia zeylanica (bruguiere, 1789) from mudasalodai, southeast coast of India, Int J Pharm Pharm Sci Res, vol. 4, p. 60-64.
- Vino AB, Ramasamy P, Shanmugam V, Shanmugam A (2012) Extraction, characterization and in vitro antioxidative potential of chitosan and sulfated chitosan from Cuttlebone of Sepia aculeata Orbigny, 1848, Asian Pac J Tro Biomed., vol. 2, p. S334-S341.
- Viruly L, Suhartono M.T, Nurilmala M, Saraswati S, Andarwulan N (2022) Identification and characterization of antimicrobial peptide (AMP) candidate from Gonggong Sea Snail (Leavistrombus turturella) extract, J. Food Sci Tech., p. 1-9.
- Wang D, Lipard S.J (2005) Cellular processing of platinum anticancer drugs, Nat rev Dru discov, vol. 4, p. 307-320.
- Xie Y, Wright S, Shen Y, Du L (2012) Bioactive natural products from Lysobacter, Nat prod rep, vol. 29, p. 277-1287.
- Yan EB, Unthank J.K, Castillo-Melendez M, Miller S.L, Langford S.J, et al. (2005) Novel method for in vivo hydroxyl radical measurement by microdialysis in fetal sheep brain in utero, J App Physio., vol. 98, p. 2304-2310.
- Yen GC, Chen H.Y, (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity, J agric food chem., 1995, vol. 43, p. 27-32.
- Yildirim A, Mavi A, Oktay M, Kara AA, Algur O.F, et al. (2000) Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea Desf Ex DC), sage (Salvia triloba L.) and black tea (Camellia sinensis) extracts, J Agric Food Chem, vol. 48, p. 5030–5034.
- Zapata A, Amemiya CT (2000) Phylogeny of lower vertebrates and their immunological structures (University of Washington Library).
- Zenebe T, Ahlgren G, Boberg M (1998) Fatty acid content of some freshwater fish of commercial importance from tropical lakes in the Ethiopian Rift Valley, J fish biol, vol. 53, p. 987-1005.
- Zhang Y, Zhou Q, Ke C.H, Huang H.Q (2014), Cloning and expression analysis of γ-aminobutyrate type A receptor-associated protein (GABARAP) in Asiatic hard clam, Meretrix meretrix, Aquaculture, vol. 432, p. 327-335.


