Assessment of Anti-Cancer Properties of Chlorella sorokiniana Against A375, A549 and HeLa Cell Lines Using GC-MS Analysis and MTT Assay Gayathri Parimalachelvam, Narasimhan Nagendran, Sankaran Balakrishnan*
Department of Plant Biology and Plant Biotechnology, Presidency College, Chennai, Tamilnadu, India
*Correspondence to: Sankaran Balakrishnan
Citation: Parimalachelvam G, Nagendran N, Balakrishnan S (2023) Assessment of anti-cancer properties of Chlorella sorokiniana against A375, A549 and HeLa cell lines using GC-MS analysis and MTT assay. Sci Academique 4(1): 55-71
Received: 13 June, 2023; Accepted: 1 July 2023; Publication: 06 July 2023
Abstract
Chlorella sorokiniana is an easily available, accessible, affordable and culturable microalga that is utilised in various industries such as nutraceuticals, biofuel and waste water treatment. This study aims to investigate the phytochemical profile and assess the anti-cancer activity of Chlorella sorokiniana. The algal sample was subjected to phytochemical extraction by cold percolation using a mixture of ethanol and water (ethanol: water, 90:10). The extract of Chlorella sorokiniana was analysed using Gas Chromatography Mass Spectroscopy (GC-MS) and the anticancer activities of the same extract was assessed using MTT assay in A375, A549 and HeLa cell lines. The GC-MS analysis identified 13 active biochemical components, which are, 1,2-Benzenedicarboxylic acid, ethyl methyl ester, 6,10,14-Trimethyl-2-pentadecanone, 9-Octadecenal, 9-Octadecenoic acid Methyl ester, Diethyl Phthalate, Dimethyl phthalate, Dotriacontane, Hexadecanoic acid methyl ester, Methyl Stearate, Phthalic acid 5-methylhex-2-yl ethyl ester, Phthalic acid ethyl pentyl ester, Tetracosane and Tetrapentacontane. Among these Tetrapentacontane, Dotriacontane and Tetracosane were found in higher concentration. The results were compared with earlier studies on the same species and confirmed that these 13 compounds were different from the previously identified compounds. The MTT assay in A375 and HeLa cell line confirmed that the viability of cancer cells is marginally affected by the extract at the concentration above 100 µg/ml and the MTT assay in A549 cell lines shows that the extract is very effective and the IC50 value is at 41.49µg/ml. These findings prove that Chlorella sorokiniana could be an affordable promising source of drug for cancer treatment and further studies on this could possibly lead to discovery of a new drug.
Keywords: Chlorella sorokiniana; GC-MS; MTT assay; Nutraceuticals; Anti-cancer; Waste water
Introduction
The term “Algae”, refers to a polyphyletic group of thalloid photosynthetic organisms. Chlorella sorokiniana is a species of microalga belonging to the Division Chlorophyta. It is an easily available, accessible, affordable and culturable microalga which is used in various industries viz., nutraceuticals, biofuel and waste water treatment, etc. It is highly rich in nutrients and is consumed as single cell protein and dietary supplements all around the world. Chlorella is rich in vitamins and minerals and it can be used as antioxidant supplements for patients undergoing chemotherapy to reduce the side effects caused thereafter [5,27]. Antioxidants level are modulated using Chlorella vulgaris extract [24]. Plant based natural antioxidants are effective than synthetic antioxidants and avoids the after effects of synthetic oxidants on liver and lungs [18]. Cellular damage caused by free radicals is prevented by natural anti-oxidants [19].
Carotenoids are antioxidants which can scavenge reactive oxygen species (ROS). Microalgae possess high amount of carotenoids which has antioxidant potential [8,23]. Algae are rich in polyphenols which can be a remedy for cancer, inflammation, diabetes, etc. The polyphenols trap the reactive oxygen species using their phenol rings [28]. Microalgae consist of a variety of phytochemicals, most of them being unexplored, gives us a wonderful opportunity of utilization. These phytochemicals have shown to be of tremendous use to man due to their antidiabetic, antimicrobial, anthelminthic, antifungal and anticancer properties. The most common microalgae such as Chlorella and Scenedesmus have been recognized as potential source for commercial cultivation for their bioactive components [3,15,16]. Theextracts derived from Chlorella vulgaris have shown toxicity against cervical cancer [27]. Chlorella pyrenoidosa extract has antiproliferative potential [10]. The PUFA rich extracts of Chlorella pyrenoidosa and Spirulina platensis have exhibited antidiabetic potential [25].
According to the World Health Organization (WHO), Cancer is second largest reason for deaths caused by non-contagious diseases. In the year 2018, 10 million have died worldwide due to cancer. According to the International Agency for Cancer Research, 19.3 million cases of cancer were reported in 2020, and by 2040, that figure is expected to rise by 47% to 28.4 million cases [7]. The three cancer kinds that account for the bulk of instances worldwide are breast, lung, and colorectal cancers [2].
Gas Chromatography Mass Spectroscopy (GC-MS) is a commonly used reliable method of phytochemical analysis, and has been adopted as the method of analysis for this study. MTT assay is one of the most efficient methods of testing the viability of cells for testing anticancer activities of phytochemicals [6]. This study aims to investigate the phytochemical profile and anticancer activities of the ethanol extract of freshwater microalgae Chlorella sorokiniana that was sourced from Kosasthalaiar one of the major river flowing through Chennai, the capital of Tamil Nadu, India.
Materials and Methods
Collection
The algal sample was collected from the Kosasthalaiar [Lat.13.208028°N: Long.80.271585°E] river using a phytoplankton net and was stored in a sterile container along with the water from the river.
Culture and Isolation
The sample was then cultured in lab using Bold Basal Medium (BBM). The pure culture was obtained using serial dilution and quadrant streak plate methods. The pure culture was identified as Chlorella sorokiniana by gene sequencing of 18s rRNA. The sample was then mass cultured in 25 litre containers.
Phytochemical extraction
The culture was centrifuged; the pellets were dried and powdered. The crude sample was mixed with aqueous ethanol (ethanol: water at ratio of 90:10) and was kept in water bath for 8 hours, then filtered using Whatman filter paper and was defatted using n-hexane. Then it was used in further studies.
Gas Chromatography Mass Spectrum (GC-MS) Analysis
The phytochemical of the algal sample was analysed using GC-MS. A GC-MS equipment (GC MS QP2020; SHIMADZU) comprising of an AOC-20s auto-sampler, an AOC-20i auto-injector and a gas chromatograph (GC-2010) interfaced to a Mass Spectrometer was used. The column used was SH-Rxi-5Sil-MS capillary standard non-polar column (Dimension: 30.0 m, Diameter: 0.25 mm, Film thickness: 0.25 µm is composed of 100% Dimethyl polysiloxane). An electron ionization energy system with ionization energy of 70eV was used. Helium gas (99.99%) was used as the carrier gas at a constant flow rate of 1.20ml/min and an injection volume of 5 µl was employed (split ratio: 10). Injector temperature 250 °C: Ion-source temperature 200 °C. The oven temperature was programmed at 50 °C (isothermal for 2 min.), to gradually increase to 280 °C in 10 minutes. Mass spectra were taken at 70eV; a scan interval of 0.3 seconds with scan range of 50 – 500 m/z. Total GC running time was 21 min. The relative percentage amount of each component was calculated by comparing its average peak area to the total areas. Shimadzu GC-MS real time analysis software was adopted to handle mass spectra and chromatograms. The mass spectrums were compared with the spectrums of the known components stored in the NIST14 (National Institute of Standard and Technology) and WILEY8 libraries.
MTT assay [20]
The anti-cancer activity of Chlorella sorokiniana was assessed using MTT assay, serial two-fold dilutions (3.125-100 µg) of the sample was prepared.
A375, A549 and HeLa cell lines were sourced from National Centre for Cell Science (NCCS), stock cell was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% inactivated Fetal Bovine Serum (FBS), 100 IU/ml of penicillin and 100 μg/ml of streptomycin in a humid atmosphere of 5% CO2 at 37 oC until confluent to obtain a monolayer cell culture.
The monolayer cell culture was trypsinized and the culture was diluted to 1.0 x 105 cells/ml using medium containing 10% FBS. 100μl of the diluted cell suspension was added to each well of the 96 well microtiter plate such that there are 1 x 104 cells/well. After 24 hours, when a partial monolayer was formed, the supernatant was removed, washed with medium and 100 μl of 3.125 µg/ml, 6.25 µg/ml, 12.5 µg/ml, 25 µg/ml, 50 µg/ml, 100 µg/ml concentrations of test samples were added on to that. The plate was then incubated at 37 oC for 24 hours in 5% CO2 atmosphere. The test solutions were discarded from the well and 20 μl of MTT (2 mg/1 ml of MTT in PBS) was added to each well. The plate was incubated for 4 hours at 37 oC in 5% CO2 atmosphere. After the removal of supernatant, 100 μl of DMSO was added and the plate was gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 570 nm for all the cell lines. The percentage of viability was calculated using the following formula.
Percentage of viability = Sample absorbance/Control absorbance x 100
Statistical analysis
ANOVA and other suitable statistical tests were conducted R Studio software and observations that were insignificant were rejected.
Results
The results of the study are as follows –
Gas Chromatography Mass Spectrum
The GCMS analysis identified thirteen compounds from the extract. Those are: 1,2-Benzenedicarboxylic acid, ethyl methyl ester (Figure 2. C11H12O4), 6,10,14-Trimethyl-2-Pentadecanone, (Figure 3. C18H36O), 9-Octadecenal (Figure 4. C18H34O), 9-Octadecenoic acid, methyl ester (Figure 5. C19H36O2), Diethyl Phthalate (Figure 6. C12H14O4), Dimethyl Phthalate (Figure 7. C10H10O4), Dotriacontane (Figure 8. C32H66), Hexadecanoic acid, methyl ester (Figure 9. C17H34O2), Methyl Stearate (Figure 10. C19H38O2), Phthalic acid, 5-methylhex-2-yl ethyl ester Figure 11. C17H24O4), Phthalic acid, ethyl pentyl ester (Figure 12. C15H20O4), Tetracosane (C24H50) and Tetrapentacontane (Figure 13. C54H110).
The analysis shows the presence of eleven major peaks. The respective retention times (Rt) of individual peak recorded were 17.15, 18.65, 20.03, 21.71, 24.66, 25.97, 26.06, 29.25, 29.25, 33.185 and 34.51. The major phyto-constituents in the fraction were Tetrapentacontane, Dotriacontane and Tetracosane. The results are shown in Table 01 and Figure 01.
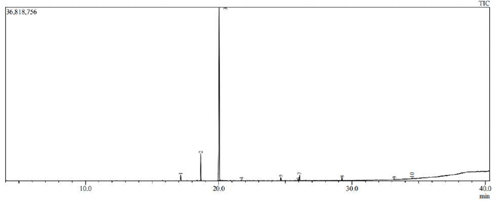
Figure 1: Retention time and peak level based on GC-MS analysis.
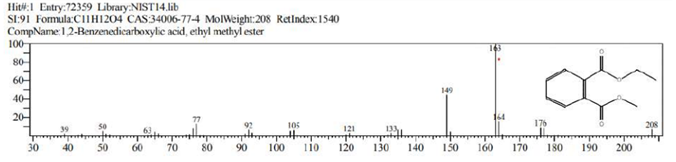
Figure 2: 1,2-Benzenedicarboxylic acid, ethyl methyl ester (C11H12O4).
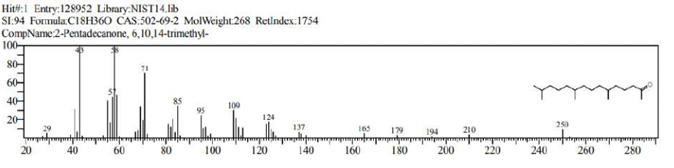
Figure 3: 2-Pentadecanone, 6,10,14-trimethyl- (C18H36O).
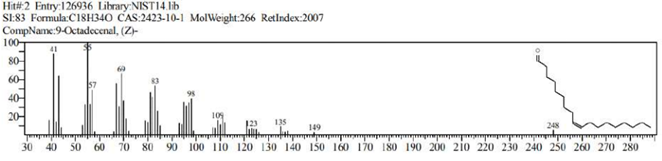
Figure 4: 9-Octadecenal (C18H34O).
Figure 5: 9-Octadecenoic acid, Methyl ester (C19H36O2).
Figure 6: Diethyl Phthalate (C12H14O4)
Figure 7: Dimethyl phthalate (C10H10O4).
Figure 8: Dotriacontane (C32H66).
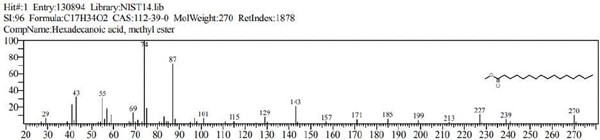
Figure 9: Hexadecanoic acid, methyl ester (C17H34O2).
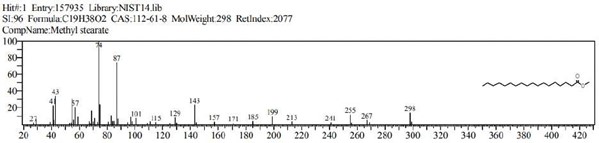
Figure 10: Methyl stearate (C19H38O2)
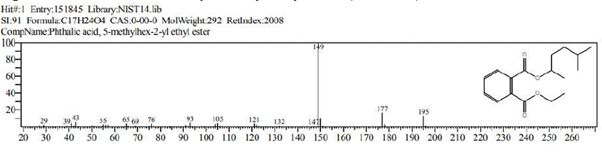
Figure 11: Phthalic acid, 5-methylhex-2-yl ethyl ester (C17H24O4)
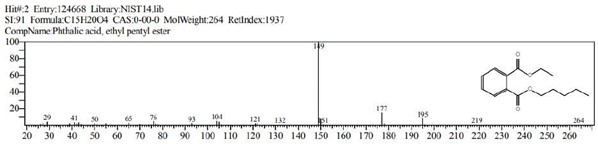
Figure 12: Phthalic acid, ethyl pentyl ester (C15H20O4)
Figure 13: Tetracosane (C24H50).
Figure 14: Tetrapentacontane (C54H110)
MTT assay
The viability of A375, A549 and HeLa cancer cell lines at 100 µg/ml concentration of test solutions are 57.96%, 29.16% and 53.98% respectively. The viability of cancer cells in different concentration of the test solutions were as given in [Table 1-4]. These observations were used to identify the inhibitory concentration mean (IC50), the observed IC50 for C. sorokiniana extract in A375 and HeLa cell lines is at concentrations above 100µg/ml, whereas the IC50 value of C. sorokiniana extract in A549 is 41.49 µg/ml. The IC50 values for Cisplatin in A375, A549 and HeLa are 6.82 µg/ml, 5.55 µg/ml and 4.17µg/ml respectively. The viability of cells in cell lines A375, A549 and HeLa at different concentrations against is shown in Photos 18, 19 and 20 and change in percentage of viability in comparison with Cisplatin are shown in Graphs 15, 16 and 17.
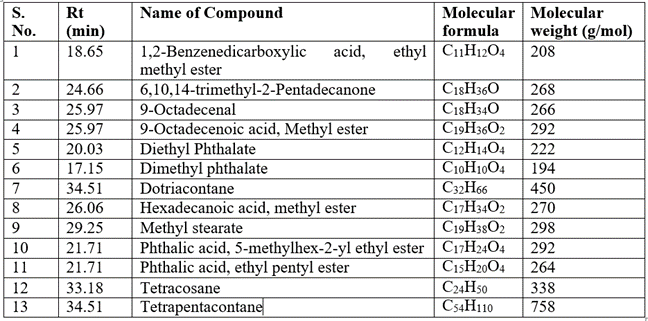
Table 1: GC-MS Analysis of Chlorella sorokiana.
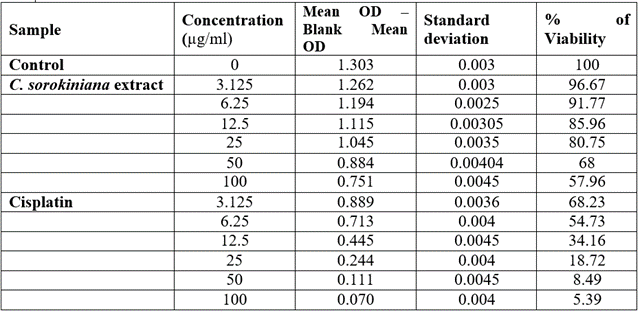
Table 2: Viability of A375 cells treated with Chlorella sorokiniana and Cisplatin
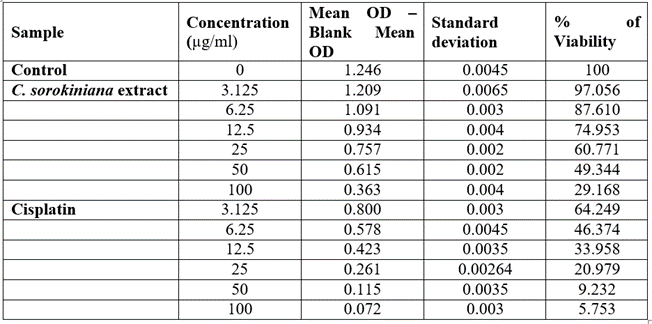
Table 3: Viability of A549 cells treated with Chlorella sorokiniana and Cisplatin.
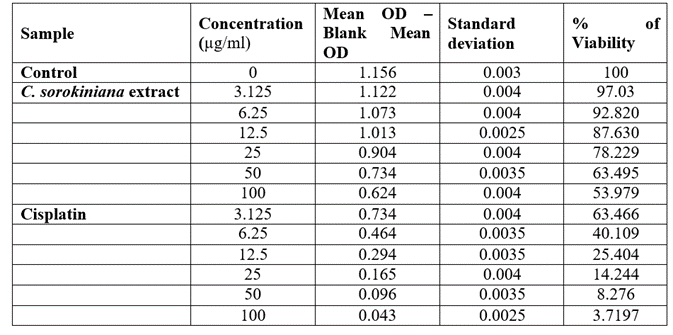
Table 4: Viability of HeLa cells treated with Chlorella sorokiniana and Cisplatin.
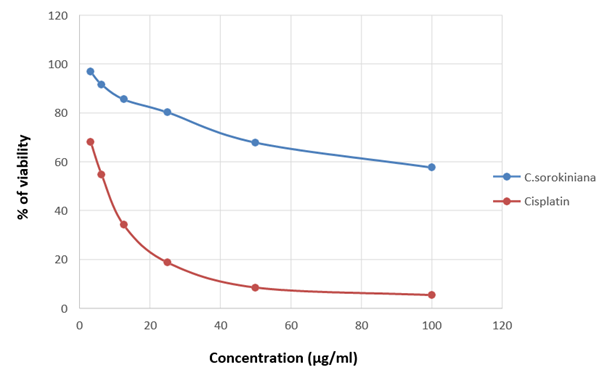
Figure 15: Graph showing the change in percentage of viability along with the concentration of the sample in A375
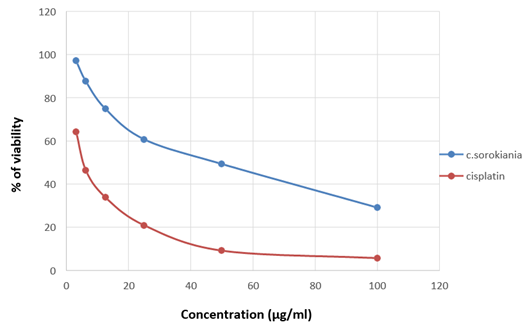
Figure 16: Graph showing the change in percentage of viability along with the concentration of the sample in A549.
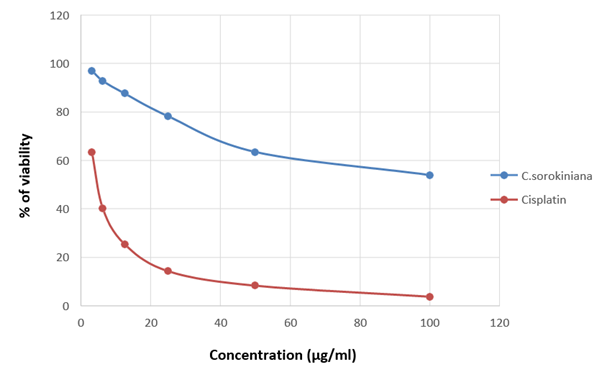
Figure 17: Graph showing the change in percentage of viability along with the concentration of the sample in HeLa.
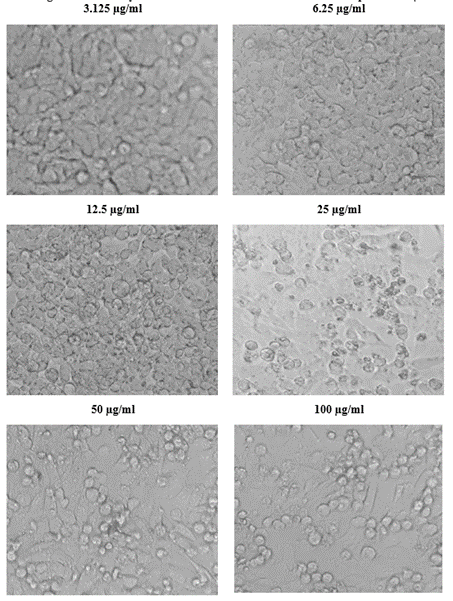
Figure 18: Viability of cells at different concentration of the sample in A375.
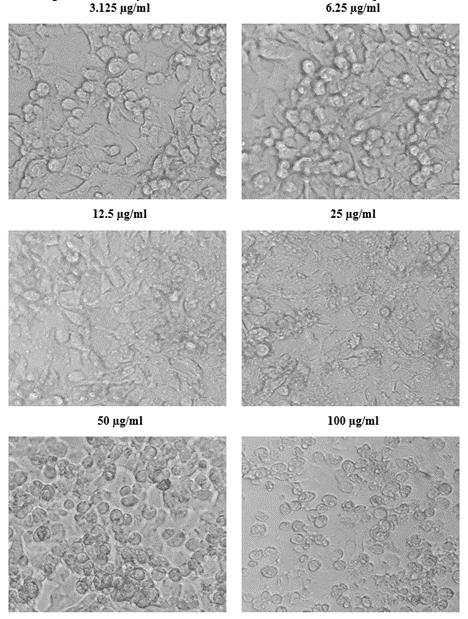
Figure 19: Viability of cells at different concentration of the sample in A549.
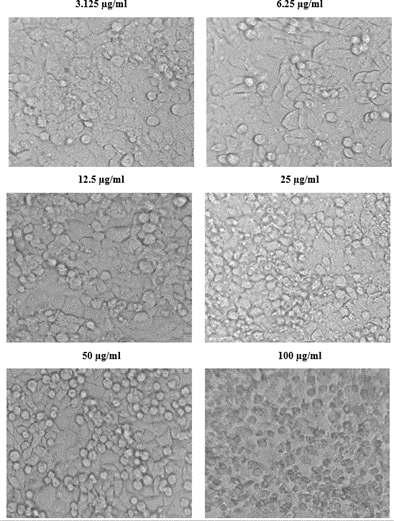
Figure 20: Viability of cells at different concentration of the sample in HeLa.
Discussion
In recent days microalgae are being used in almost all industries for various purposes. Among them, multiple species of Chlorella are the top choices for almost all the industries. The metabolic pathways and the phytochemical profile of microalgae are largely unexplored. The exploration of these phytochemical components could be a solution to the rise in antimicrobial resistance and cancer.
The GC–MS analysis of cyanobacteria, Phormidium fragile (Cyanobacteria) has showed presence of 27 compounds [13]. Few dominant compounds in the extract were 8-Octadecanoic acid methyl ester and Hexadecanoic acid methyl ester. The GC-MS analysis of Chlorococcum humicola revealed the presence of 5 compounds and one among them is 6,10,14-trimethyl-2-Pentadecanone. These chemicals have high therapeutic potential against microbes, cancer, etc. [1].
The GCMS analysis of Chlorella sorokiniana in methanol extract previously done by [21] identified the presence of 34 components. The major components identified includes (R)-(-)-2-hexanol, n-hexadecanoic acid and octadecanoic acid. The present study identified 13 different bioactive compounds which were found to be exclusive from the results of the previous studies. This elucidates the fact that this particular species has a diverse phytochemical profile. The antibacterial and antifungal activity of 6, 10, 14- trimethyl-2-pentadecanone, a compound identified in this extract was explored by [26].
According to the report of WHO, cancers account for 10 million deaths annually. melanoma, lung adenocarcinoma and cervical cancer are some of the most prevalent cancers in current times and there is a requirement of better drugs to treat them. The previous studies on anticancer properties of Chlorella sorokiniana state that Cytotoxicity of C. sorokiniana methanol extract in L5178Y-R tumour cell line resulted in 62–75% toxicity at 500 µg/ml concentration [17] and the previous study by [22] shows that cell wall membrane fraction from the cell membrane fraction of C. sorokiniana inhibits the growth of murine and human colon carcinoma cells and study by [9] shows that the extract of the same species is effective against hepatocellular carcinoma in Hep G2 cell line. Chlorella sorokiniana as food supplement has reduced the release of reactive oxygen species in to the mitochondria of rat liver [14]. Colon carcinoma growth is inhibited by the bioactive compound present in the cell wall of Chlorella sorokiniana [22].
The present study shows that the viability of cancer cells under the influence of 100 µg/ml concentration of test solutions reduce to 57.96%, 29.16% and 53.98% in A375, A549 and HeLa cell lines, proving that the ethanol extract of Chlorella sorokiniana is effective against melanoma, lung adenocarcinoma and cervical cancer. Comparing the other studies, this study shows better antiproliferation in a lower concentration suggesting that the ethanol extract could have better anticancer abilities than other extracts. This extract shows better effect on A549 than other cell lines suggesting that this extract is more effective towards lung adenocarcinoma.
Anticancer compounds can induce apoptosis in specific cells [4]. Any one of the compound from Chlorella sorokiniana can induce programmed cell death [11]. The anticancer activity of this extract could be because of either one of the 13 compounds or due to a combination of two or more compounds, fractioning this extract further and investigating those fractions and their anticancer activities could help us narrow down the compound helping us to find an easier method of extraction and a better cure. Chlorella sorokininana extract can be a chemo protector against cisplatin-induced bone marrow toxicity [12].
Conclusion
The current study showed that easily available microalga Chlorella sorokiniana has major phytochemicals such as Tetrapentacontane, Dotriacontane and Tetracosane. Its extraction is effective against A375, A549 and HeLa cell line, it shows IC50 concentration of A375 and HeLa cell lines are above 100 µg/ml whereas A549 is 41.49µg/ml. Further the outputs of its activity profile, C. sorokiniana have been recommended as a promising source of anticancer drug especially against lung adenocarcinoma.
References
- Balaji M, Thamilvanan D, Vinayagam SC, Balakumar BS (2017) Anticancer, antioxidant activity and GC-MS analysis of selected micro algal members of Chlorophyceae. International journal of Pharmaceutical Sciences and Research. 8: 3302-14. doi: 10.13040/IJPSR.0975-8232.8(8).3302-14.
- Cotas J, Pacheco D, Gonçalves AMM, Silva P, Carvalho LG, et al. (2021) Seaweeds’ nutraceutical and biomedical potential in cancer therapy: a concise review. Journal of Cancer Metastasis and Treatment. 7: 13. http://dx.doi.org/10.20517/ 2394 -4722.2020.134.
- Dolganyuk V, Belova D, Babich O, Prosekov A, Ivanova S, et al. (2020) Microalgae: A promising source of valuable bioproducts. Biomolecules 10: 1153. https://doi.org/10.3390/biom10081153.
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicologic Pathology: 35. DOI 10.1080/01926230701320337.
- Ferdous Umme Tamanna, Yusof Zetty Norhana Balia (2021) Medicinal Prospects of Antioxidants from Algal Sources in Cancer Therapy. Frontiers in Pharmacology. 12.
- Ghasemi M, Turnbull T, Sebastian S, Kempson I (2021) The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int J Mol Sci 22: 12827. doi: 10.3390/ijms222312827.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A cancer journal for clinicians 71: 209-249. https://doi.org/10.3322/caac.21660
- Jahnke L (1999) Massive carotenoid accumulation in Dunaliella bar- dawil induced by ultraviolet-A radiation. Journal of Photochemistry and Photobiology. 48: 68–74.
- Chung JG, Peng HY, Chu YC, Hsieh YM, et al. (2012) Anti-invasion and apoptosis induction of chlorella (Chlorella sorokiniana) in Hep G2 human hepatocellular carcinoma cells. Journal of functional foods. 4: 302 – 310. https://doi.org/10.1016/j.jff.2011.12.008.
- Kyadari M, Fatma T, Azad R, Velpandian T (2013) Evaluation of antiangiogenic and antiproliferative potential of the organic extract of green algae Chlorella pyrenoidosa. Indian Journal of Pharmacology 45: 569_574. DOI 10.4103/0253-7613.121366.
- Lin PY, Tsai CT, Chuang WL, Chao YH, Pan IH, et al. (2017) Chlorella sorokiniana induces mitochondrial-mediated apoptosis in human non-small cell lung cancer cells and inhibits xenograft tumor growth in vivo. BMC Complement Altern Med 17: 88. doi: 10.1186/s12906-017-1611-9.
- Lin SH, Li MH, Chuang KA, Lin NH, Chang CH, et al. (2020) Chlorella sorokiniana Extract Prevents Cisplatin-Induced Myelotoxicity In Vitro and In Vivo. Oxid Med Cell Longev 25: 7353618. doi: 10.1155/2020/7353618.
- Mukund S, Sivasubramanian V, Senthilkumar NS (2014) In-silico studies on metabolites of Phormidium fragile against colon cancer EGFR protein. Journal of Algal Biomass Utilization 5: 16- 22.
- Napolitano G, Fasciolo G, Salbitani G, Venditti P (2020) Chlorella soro–kiniana Dietary supplementation increases antioxidant capacities and reduces ROS release in mitochondria of hyperthyroid rat liver. Antioxidants 9: 883.
- Pantami HA, Bustamam A, Lee MS, Ismail SY, Mohd Faudzi IS, et al. (2020) Comprehensive GCMS and LC-MS/MS Metabolite Profiling of Chlorella vulgaris. Marine Drugs 18: 367. https://doi.org/10.3390/md180703673.
- Patil L, Kaliwal BB (2019) Microalga scenedesmus bajacalifornicus BBKLP-07, a new source of bioactive compounds with in vitro pharmacological applications Bioprocess Biosystms Engineering 42: 979-94. https://doi.org/10.1007/s00449-019-02099-53.
- Reyna-Martinez R, Gomez-Flores R, López-Chuken U, Quintanilla-Licea R, Caballero-Hernandez1 D, et al. (2018) Antitumor activity of Chlorella sorokiniana and Scenedesmus sp. microalgae native of Nuevo León State, México. Peer J 6: e4358; DOI 10.7717/peerj.4358
- Roy N, Laskar RA, Ki S, Kumari D, Ghosh T, et al. (2011) A detailed study on the antioxidant activity of the stem bark of Dalbergia sissoo Roxb., an Indian medicinal plant. Food Chemistry 126: 1115-21. https://doi.org/10. 1016/j.foodchem.2010.11.143.
- Salman KA, Ashraf S (2015) Reactive oxygen species: a link between chronic inflammation and cancer. Asia Pacific Journal of Molecular Biological & Biotechnology 21: 42-49.
- Sodde VK, Lobo R, Kumar N, Maheshwari R, Shreedhara CS (2015) Cytotoxic activity of Macrosolen parasiticus (L.) Danser on the growth of breast cancer cell line (MCF-7). Pharmacognosy magazine 11: S156.
- Songserm R, Kaeoboon S, Suksungworn R, Duangsrisai S, Sanevas N (2021) GC-MS profiling, anti-oxiant an anti-diabetic assessments of extracts from microalgae Scenedesmus falcatis (KU.B1) and Chlorella sorokiana (KU.B2). Plant Science Today 9: 632-641.
- Susumu Ishiguro, Nicole Robben, Riley Burghart, Paige Cote, Sarah Greenway, et al. (2020) Cell Wall Membrane Fraction of Chlorella sorokiniana Enhances Host Antitumor Immunity and Inhibits Colon Carcinoma Growth in Mice. Integrative Cancer Therapies 19: 1–10.
- Takaichi S (2011) Carotenoids in algae: distribution biosynthesis and functions. Marine Drugs. 9: 1101–1118.
- Vijayavel K, Anbuselvam C, Balasubramanian MP (2017) Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Molecular and Cellular Biochemistry 303: 39-44.
- Wan XZ, Li TT, Zhong RT, Chen HB, Xia X, et al. (2019) Anti-diabetic activity of PUFAs-rich extracts of Chlorella pyrenoidosa and Spirulina platensis in rats. Food and Chemical Toxicology 128: 233-39. https://doi.org/ 10.1016/j.fct.2019.04.017
- Yasukawa K, Akihisa T, Kanno H, Kaminaga T, Izumida M, et al. (1996) Inhibitory effects of sterols isolated from Chlorella vulgaris on 12-o- tetradecanoyl phorbol-13-acetate-induced inflammation and tumor promotion in mouse skin. Biological and Pharmaceutical Bulletin 19: 573-576.
- Yusof Y, Saad S, Makpol S, Shaaman N, Ngah W (2010) Hot water extract of Chlorella vulgaris induced DNA damage and apoptosis. Clinics 65: 1371-1377. DOI 10.1590/S1807-59322010001200023.
- Zakaria NA, Ibrahim D, Sulaiman FS, Supardy NA (2011) Assessment of antioxidant activity, total phenolic content and in- vitro toxicity of Malaysian red seaweed, Acanthophora spicifera. Journal of Chemical and Pharmaceutical Research. 3: 182-19.


