The Role of Optimized Diet in Periodontal Health Maintenance Shreya Kohir, Reshma Suresh*, Maya Rajan Peter, Biju Balakrishnan, Anjali R Nath, K.V. Arun
Department of Periodontics and Implantology, Amrita School of Dentistry, Amrita Vishwavidhyapeetham, Kochi, Kerala
*Correspondence to: Dr. Reshma Suresh
Citation: Kohir S, Suresh R, Peter MR, Balakrishnan B, Nath AR, et al. (2023) The Role of Optimized Diet in Periodontal Health Maintenance. Sci Academique 4(2): 116-131
Received: 06 November, 2023; Accepted: 23 November, 2023; Publication: 29 November, 2023
Abstract
Food habits have almost a direct link to maintaining a good oral hygiene. Diet and nutritional health are thought to be as modifiable lifestyle risk factors for the development of periodontal disease. Latest studies have shown about the developed food habits, distinguished majorly by convenience foods (processed carbohydrates such as sugar and plain flour, elaidic acids and low micronutrient densities), might be involved in promoting periodontitis. On the other hand, low blood glucose and compound carbohydrates (found in vegetables, fruits and legumes), polyunsaturated fatty acids, microelements such as vitamins and minerals etc., phytonutrients, nitrates of plant origin, and fibers are also considered important for the management of oral health. Following a proper diet plan, consuming calorie restricted diet and following intermittent type of fasting regimes have also shown promising results in minimizing periodontal inflammation and also result in overall maintenance of oral cavity. This review focuses on how optimized diet along with calorie restriction and intermittent fasting regimes will be helpful in influencing the maintenance of periodontal health and overall oral health.
Keywords: Optimized diet; Calorie restriction; Periodontal health; Periodontal diseases; Intermittent fasting, Oral health
Introduction
Periodontal disease, or periodontitis is basically the swelling of the periodontal ligament and the tissues surrounding which in most cases is followed by attachment and alveolar bone loss surrounding the teeth. It is one of the major and frequent oral health related diseases affecting around 74.3 Cr individuals around the entire world and is also a major reason for loss of teeth amongst elderly [1,2]. In late 60s periodontitis was thought to be mainly, a plaque-induced disease according to Löe et al [3]. In the due course of assessing its etiology or origin, distinctive speculations were made over few years which gave a better and clearer picture about the disease presence and progression. Marsh and Devine proposed a “The Ecological Plaque Hypothesis” [4], wherein they stated that the periodontal microbes or bacteria are advocated and aided by the inflammatory response of the host, which not only increases gingival febrility, but along with poor nutrition might contribute to the increase in the amount of secreted gingival exudate. Baumgartner and colleagues [5], conducted research evaluating different individuals during an archaic analysis, revealing noticeable decline of gingivitis and periodontitis, even when oral hygiene was not very well maintained.
Periodontal disease Pathogenesis
Periodontal health is mainly controlled by the maintenance of homeostasis in the immunity and the microbiota which is symbiotic. Periodontitis occurs most commonly in relation with a community which is dysbiotic and polymicrobial wherein different individual microbes have different and varying roles that help in inflammation which in most cases can is destructive. Keystone pathogens like P.gingivalis along with the dysbiotic bacteria like Prevotella intermedia and Aggregeticbacteractinomycetemcomitans cause a positive feedback loop which might further aid in dysbiosis resulting in inflammation finally resulting in periodontal disease. The risk factors include immunological disorders, poor nutrition, smoking, etc. [6].
Balanced diet
A balanced diet can be defined as a “pattern of food intake that has beneficial effects on health” [7]. Since a few years, experimental results have shown a satisfactory effect of nutrient rich food plans on dental well-being. A variety of range of macro and micro-nutrients might have some influence upon healthy periodontium. The balanced consumption of un-processed compound polysaccharides, plant based amino-acids, Ω-three fatty acids, minerals, and vitamins have a positive impact on periodontal inflammation which is usually followed by disease. Hence, it shows that choosing a correct lifestyle and following proper diet habits must be encouraged in individuals with dental and periodontium related diseases [8]. Table no 1 shows various studies performed with respect to nutrients and various diet components.
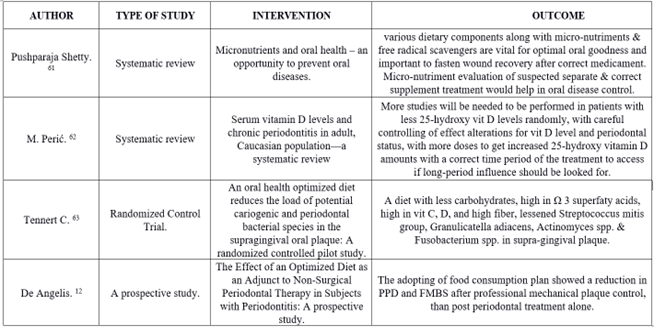
Table 1: Shows various studies performed with respect to nutrients and various diet components.
Importance of diet consistency
An unhealthy or poor diet has been implicated as a risk factor for several chronic diseases that are known to be associated with oral diseases. Dentistry has an important role in the diagnosis of oral diseases correlating with diet. Consistent healthy nutrition intake guidelines are essential to improve health overall. A poor diet was significantly associated with increased levels of oral disease. Dietary advice for the prevention of oral diseases has to be a part of the routine patient education practices [9].
Foods to avoid to maintain a good oral health [10]
- Sticky candies and sweets
- Starchy foods that can get stuck in your mouth.
- Carbonated soft drinks.
- Substances that dry out your mouth
Optimized diet
Optimal diet is the “is the ratio of macro and micronutrient food intake, or the quantity of calories given in by fat, carbohydrate, and protein consumption” [11]. Food consumption relied on compound polysaccharides or simply compound carbohydrates are normally healthy, although the foods high in refined polysaccharides can be related with increased tenderness of the oral soft tissue. Exaggerated surges in blood sugar levels (glucose) post food-consumption seems to increase in calorie rich foods, mainly the foods which are refined and processed with high sugars and fats that can be dissolved fast into the blood [12]. The components of optimized diet are mentioned in the figure 1.
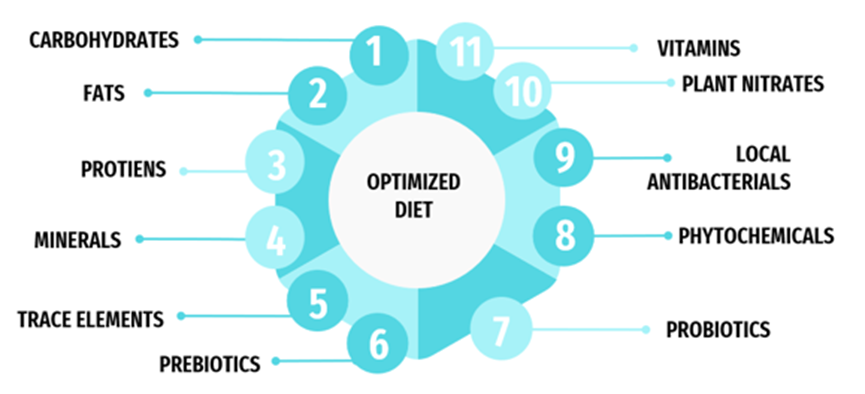
Figure 1: Components included in optimal diet.
| CALORIES | 2671 kcal |
| CARBOHYDRATES | 267 grams |
| PROTIENS | 90 grams |
| FATS | 30 grams |
| FIBERS | 35 grams |
| WATER | 2.50 -3 litre |
| ZINC | 17 milligrams |
| IRON | 19 grams |
| VITAMIN C | 18 grams |
| VITAMIN D | 600 IU |
| Kcal = kilocalories. IU = international unit. | |
Table 2: Lists the Optimized diet elements and their quantities.
Intermittent fasting
It is a diet regime that needs an individual to abstain from consuming food items or beverages for varying periods of time; for instance, a 16:8 fasting regime (i.e., a sixteen-hours fasting with an eight-hours eating window period), twenty-four -hours fast, the 5:2 diet (i.e., regular food consumption for 5 days a week and then minimising calorie consumption to five hundred-six hundred calories on the next two days), time-restricted food consumption, and fasting every other-day. In Alternative-day type of fasting, an individual’s food intake is alternated between absence of consumption of any calories for a day and consuming food without any restriction the following day [14].
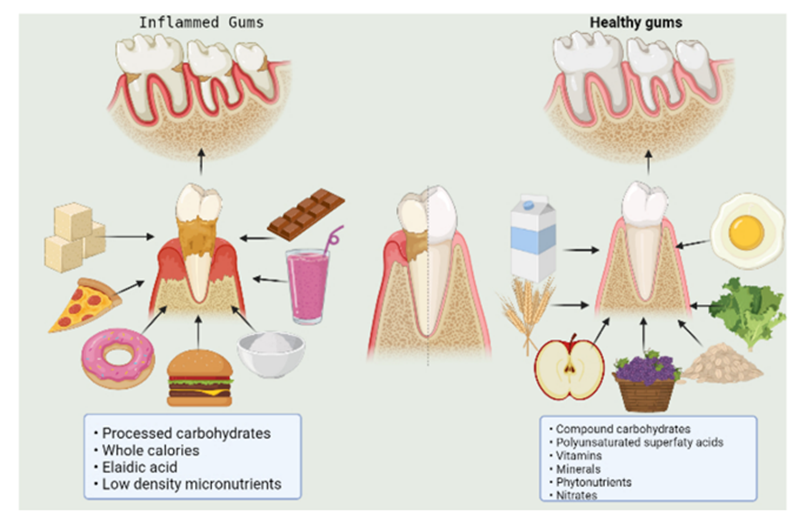
Figure 2: Diet food components and their effect on the gums and periodontium.
The Penmen derived to a conclusion that the existing guidelines for evaluating gingivitis are not relevant if dietary intake does not comprehend processed carbohydrates [15]. Hence, diet is found to have a consequence on the periodontal and gingiva related inflammatory reactions. The diet-related well-being of periodontal tissues can be seen, when carbohydrates are reduced, and an added consumption of polyunsaturated fatty acids, vitamin D, C, free-radical scavenger’s and fibre is consumed [16–22]. Also, the initial studies performed on animals give some insight into the localized and systemic effects of restricted intake of calories and intermittent type of fasting on inflammatory mediators that have influence on inflammation of the periodontium and the progression of the disease [23–27].
Role of optimized Diet in Periodontal Health
The increased consumption of polysaccharides helps in dysbacteriosis ad leads to infectious conditions [15]. The minimizing of the diet rich in polysaccharides seems to decrease gingival tenderness [16]. In-vitro researches revealed that increased sugar levels might cause cellular death and stall regeneration of periodontal ligament tissues [15]. Also, an imbalance between Ω-6 and Ω-3 fatty acids promote tenderness. Positively, there is increasing proof that Ω-3 fatty acids paves way to decrease inflammatory reactions [15]. Hence it can be said that the lesser consumption of carbohydrates or polysaccharides, and an increased consumption of Ω fatty acids helps in the reduction of the inflammation caused during periodontal diseases.
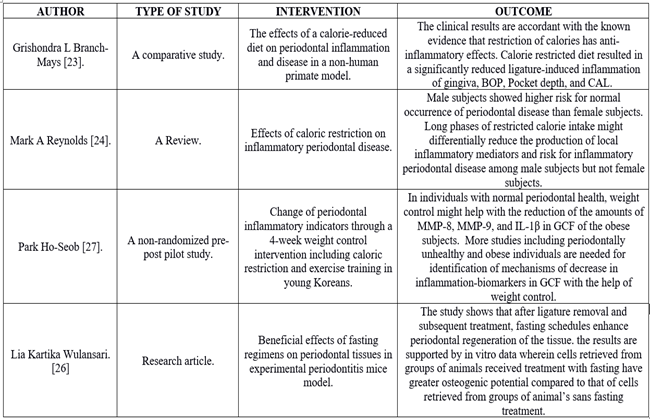
Table 3: showing components in the Optimized diet chart.
Intermittent Fasting and Calorie Restriction
Periods of deliberate abstinence from food and beverages has been practiced since early times by people around the world. Data on ethnohistory and religious texts depict various fasting regimens and methods [28]. Intermittent fasting is of various types and one has to follow a different regimen in each type. Effects of calorie restricted diet and intermittent type of fasting on the periodontium are depicted in the figure no 3.
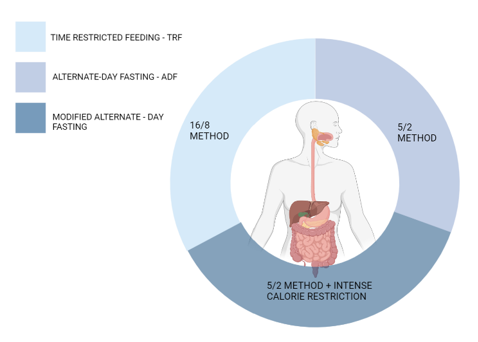
Figure 3: Types of intermittent fasting.
| Meal | Menu | Portion |
| Early morning (7:00 AM) |
warm lemon water + linseeds/ benne seeds / mirasol seeds |
1 tumbler One tbspn (15 grams) |
| After 20 minutes | ||
| Masala chai/ curcuma milk + badam/kaju/ pista/walnut | 70 millilitre / 150milliletre 10 numbers. | |
| Breakfast (9:00am) |
Veg flattened rice/ Oatmeal + 1 citrus fruit Or 3-4 Idly + Sambhar+ papaya Or 2 Boiled eggs with two brown bread slices+ apple Or Veg dal Dhaliya |
1 cup (100 grams) 1 crock+ 50 grams 1 crock (150-200grams) |
| Mid-Morning (11-12 pm) |
Banana/ avocado shake Or Green smoothies (spinach, pudina, parsley, limon). |
1 tumbler (200millilitre) 200 millilitres |
| Lunch (2-3pm) |
Vegetable mash Dal (toor/channa Rajma/ chole/soya) Leafy greens Other veggies Rice (brown/white) Multigrain chapati (If you don’t take rice then take 3 roti) yoghurt/ buttermilk |
60grams 30grams 30grams 30grams 30grams 2 number medium size 50grams/250millilitre |
| Evening (5-6pm) |
Masala chai/ muskmelon juice + Sprouts/ roasted groundnut/chickpeas (Add ½ lemon on it) | 1 cup/ 200millilitre, 50grams |
| Dinner (8pm) |
Lentil soup/ Chicken / veg broth Mix veg + chapati + yoghurt Roasted fish/ chicken lentil porridge + yoghurt + Cottage cheese Veg + rice/ chapati + yoghurt |
1 crock (150 millilitre) 30grams + 2 numbers, 30 grams 100grams 1 crock(150grams) + 50grams 50grams,30grams/1,2roti+30grams |
| Bedtime (10pm) |
curcuma / elaichi milk+ Walnut/ Pista | 150 millilitres,5-10 numbers. |
Table 4: Showing various studies done with respect to calorie restriction and intermittent fasting.
| Type of fasting | Regimen details |
| Complete alternate-day fasting | This regimen involves fasting on alternative days (avoiding consumption of energy containing foods or drinks) while consuming on the remaining days (foods and beverages consumed in limit). |
| Modified fasting regimen | This regimen allows for consumption of 20%-25% of energy containing food on scheduled fasting days. This regimen forms the base for the popularly known 5:2 regimen, which includes extreme energy restriction for two non-consecutive days every week and limited food consumption on other five days. |
| Time-restricted feeding | This regimen allows individuals for consumption of limited energy intake within specific time gaps, which induces fasting periods on a routine basis. |
Table 5: Showing Types of fasting and regimens.
Components inclusive of the optimized diet are Carbohydrates
Carbohydrates or the polysaccharides alongside fats, are large energy sources. Unlike consumables with low-carbohydrates, high-carbohydrate foods cause a faster rise in blood sugar levels [17,30,31]. A latest study showed, carbohydrate intake and an increased whole caloric consumption caused inflammatory reactions [15]. The latest studies establish that high-polysaccharide containing food consumption might escalate gingival and periodontal disease [6,15,16,32]. In disparity, the food intake which is plentiful in compound or un-processed polysaccharides and fibres is analogous with reduced occurrence of gingivitis and periodontitis [15,22,33]. Due to the noted interrelation of high-glycemic carbs and caries, a latest study group about the confines in-between cariogenicity and periodontally compromised diseases noticed refined carbohydrates as a split probability factor for either of the diseases [21].
Lipids
Lipids provide energy and power and are basis of organizational and assimilated complexes (such as cell membranes and hormonal components) [34]. Serhan and fellow workers found that, the intent of an inflammatory reaction is an active flow of events focused on metabolites of Ω-three superfaty acids, presumed as “specialized pro-resolving mediators” (S.P.M), in lieu of unassertive events based upon the eradication of the inflammatory cytokines [18].
Amino acids
Proteins on the other hand are made-up of amino acids and are found in all cells. in regard with innate inflammatory process, amino acids (proteins) are observed to be relatively unbiased [34]. Animal amino acids are believed to raise insulin-like growth factor one in the body which is detected to have a vital role in causing cancer [35–39]. On the contrary, plant amino acids might seem to lessen the probability of cardiac related diseases, type II diabetes mellitus, and related diseases [40–42]. Within the sphere of periodontology, only a few investigations have been done to assess if proteins in general or specifically animal proteins are related to periodontium associated diseases. Penmen like Osborn MO [33], Staufenbiel I [43], Zong G [44] in their investigations stated that, vegetarians showed lesser pocket depths, lesser bleeding on probing, and superior oral health maintenance in comparison with meat consuming individuals [43] along with good oral health maintenance. In an analysis of periodontal inflammatory ailments, all the patients consumed a diet abundant in amino acids [45]. Individuals with reduced serum cobalamin levels had a greater likelihood of occurrence of periodontium related lesions [44].
Vitamins
Impact of vitamins and other microelements has been in discussion since long in relation to periodontal related diseases [20]. Vitamin micronutrients can be assorted by their potentiality to break down in fats, (vitamins like retinol, phylloquinone, D2, D3 and Tocopherol) or to breakdown in water (like ascorbic acid and B-complexes). Prevalence studies manifested lesser serum levels of vitamin micronutrient C and its lesser consumption in periodontally diseased patients in comparison to patient’s sans periodontium related disease. Vitamins behave in a different way when present in plants (e.g., phytonutrients, enzymes) in comparison with the factitious structure [46,47]. Greater ingestion of vitamin C containing fruits (like Citrus paradisi, lime, bell pepper species, kiwifruit etc.) might decrease gingiva and periodontium related inflammation [15,48], and an intake two grams of unnatural ascorbic acid as a supplement to non-surgical periodontal therapy revealed scarce outcome after four weeks in a placebo-controlled experiment [49]. Krall et al and Laky et al noticed reduced tooth losses in an inactive drug-controlled randomized experimental investigation where patients (of age around 65 years) were supplemented with calciferol [50] and calcium respectively. Groups supplemented with vitamins showed a positive response compared to the placebo-controlled groups [51]. Positive outcomes on periodontium related health were noticed for cobalamins along with alpha-tocopherol and also retinol [13,20,43,52].
Minerals
Evaluative reviews and prevalence studies revealed that magnesium oxide insufficiency corresponds to greater occurrence of periodontium related disease in adults, and that calcium oxide consumption inversely corresponds with periodontal disease in Japanese females of younger age groups [29,53–55]. which means that in different areas or parts of the world, the mineral consumption is different and has certain effect on the oral tissues.
Pre and Probiotics
Supplementation of probiotics as an adjunct to regular professional mechanical plaque removal remarkably decreased gingiva related inflammation [56]. The usage of probiotics with an effectiveness record showed by clinical trials, might show a valuable effects obtainable therapeutic options, chiefly in clinical scenarios in which entrenched notions relied on plaque management cannot be executed with the needed results [57]. Pre-biotics are largely unmetabolized fibers that are made up of compound polysaccharides (like inulin and galacto-oligosaccharides). Slomka et al. found two prebiotics which might be convenient in encouraging the bacteriae, like D- mannac, methyl beta-D-galactopyranoside for oral well-being [58].
Phytochemicals, plant nitrates and local Anti-bacteria
Phytonutrients are chemical elements assimilated by flora or plants with many health profits or benefits. They are inclusive of phytochemicals (like polyphenols, catechins, ligands, anthocyanin, isoflavones etc) and carotenoids and mainly have an anti-inflammatory effect [59]. Clinical trial’s by Jockel-Schneider et al [60] revealed an everyday regular consumption of 300 mL of Lactuca sativa ( commonly knowns as lettuce) extract (with 200 milligrams of nitrates) remarkably decreased gingiva related inflammation. Apart from the expressed systemic-mediated consequences of food intake on periodontal health, there are a lot of antibacterial complexes encountered in eatables. Diet food components and their effect on the gums and periodontium are depicted and mentioned in the figure no 4.
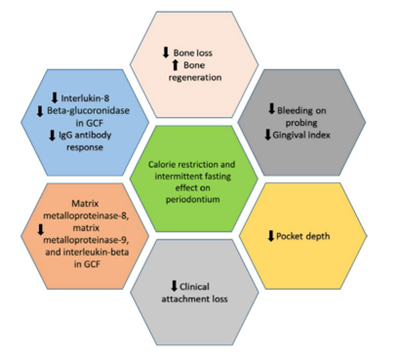
Figure 4: Effects of calorie restricted diet and intermittent type of fasting on the periodontium.
Current and future aspects
One’s diet can help in maintaining the health of the periodontium. It can be considered like prevention before the treatment. Various components mentioned in the above literature when optimized accordingly to every individual’s need might help in the overall maintenance of the body as well as the oral cavity. The optimization should be done according to the demographics of an individual and the patients should be educated and motivated to follow and keep up with the diet and the general oral hygiene. In this way we might be able to prevent the periodontal disease to a certain extent and in cases where the disease has already sent in, it can be a commendable adjunct to the dental treatment which would be provided to the patients.
Conclusion
Concluding the conferred literature, an essentially plant grounded low-carb diet, abundant in Ω-3 fatty acids, might be a classic formulation for periodontal, oral and general health. Along with the proper diet, fasting and calorie restriction in the food consumption has shown promising results in the oral hygiene maintenance. Furthermore, studies and clinical trials with much details and results need to be worked on so, as to know in depth with respect to the consumption of the particular diet components and the practicing of the diet regimens.
References
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W (2014) Global Burden of Severe Periodontitis in 1990-2010: A Systematic Review and Meta-regression. J Dent Res 93(11): 1045-1053.
- Reich E, Hiller KA (1993) Reasons for tooth extraction in the western states of Germany. Commun Dent Oral Epidemiol 21(6):379-383.
- Löe H, Theilade E, Jensen SB (1965) Experimental Gingivitis in Man. Journal of Periodontology 36(3):177-187.
- Marsh PD, Devine DA (2011) How is the development of dental biofilms influenced by the host?: Host influence on biofilm development. Journal of Clinical Periodontology 38:28-35.
- Baumgartner S, Imfeld T, Schicht O, Rath C, Persson RE, Persson GR (2009) The Impact of the Stone Age Diet on Gingival Conditions in the Absence of Oral Hygiene. Journal of Periodontology 80(5):759-768.
- Hajishengallis G, Chavakis T, Lambris JD (2020) Current understanding of periodontal disease pathogenesis and targets for host‐modulation therapy. Periodontol 2000 84(1):14-34.
- Stevenson RJ (2017) Psychological correlates of habitual diet in healthy adults. Psychological Bulletin143(1):53-90.
- Santonocito S, Polizzi A, Palazzo G, Indelicato F, Isola G (2021) Dietary Factors Affecting the Prevalence and Impact of Periodontal Disease. Clinical, Cosmetic and Investigational Dentistry 13:283-292.
- Scardina GA, Messina P (2012) Good Oral Health and Diet. J Biomed Biotechnol. 2012:720692.
- The Best and Worst Foods for Your Teeth – Health Encyclopedia – University of Rochester Medical Center, 2022.
- Koliaki C, Spinos T, Spinou Μ, Brinia ΜE, Mitsopoulou D, Katsilambros N (2018) Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthcare 6(3):73.
- De Angelis P, Gasparini G, Manicone PF, et al. (2022) The Effect of an Optimized Diet as an Adjunct to Non-Surgical Periodontal Therapy in Subjects with Periodontitis: A Prospective Study. Healthcare (Basel) 10(3):583.
- Dodington DW, Fritz PC, Sullivan PJ, Ward WE (2015) Higher Intakes of Fruits and Vegetables, β-Carotene, Vitamin C, α-Tocopherol, EPA, and DHA Are Positively Associated with Periodontal Healing after Nonsurgical Periodontal Therapy in Nonsmokers but Not in Smokers. The Journal of Nutrition145(11):2512-2519.
- Parveen S (2021) Impact of calorie restriction and intermittent fasting on periodontal health. Periodontol 2000 87(1):315-324.
- Woelber JP, Bremer K, Vach K, et al. (2017) An oral health optimized diet can reduce gingival and periodontal inflammation in humans – a randomized controlled pilot study. BMC Oral Health 17(1):28.
- Hujoel P (2009) Dietary Carbohydrates and Dental-Systemic Diseases. J Dent Res 88(6):490-502.
- Bosma-den Boer MM, van Wetten ML, Pruimboom L (2012) Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutr Metab (Lond) 9(1):32.
- Serhan CN, Chiang N, Dalli J (2015) The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Seminars in Immunology 27(3):200-215.
- Simopoulos AP (2006) Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomedicine & Pharmacotherapy 60(9):502-507.
- Van der Velden U, Kuzmanova D, Chapple ILC (2011) Micronutritional approaches to periodontal therapy: Micronutritrients and periodontal therapy. Journal of Clinical Periodontology 38:142-158.
- Chapple ILC, Milward MR, Ling‐Mountford N, et al. (2012) Adjunctive daily supplementation with encapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: a double‐blind RCT. J Clin Periodontol 39(1):62-72.
- Merchant AT, Pitiphat W, Franz M, Joshipura KJ (2006) Whole-grain and fiber intakes and periodontitis risk in men. The American Journal of Clinical Nutrition 83(6):1395-1400.
- Branch-Mays GL, Dawson DR, Gunsolley JC, et al. (2008) The Effects of a Calorie-Reduced Diet on Periodontal Inflammation and Disease in a Non-Human Primate Model. Journal of Periodontology 79(7):1184-1191.
- Reynolds MA, Dawson DR, Novak KF, et al. (2009) Effects of caloric restriction on inflammatory periodontal disease. Nutrition 25(1):88-97.
- Ebersole JL, Steffen MJ, Reynolds MA, et al. (2008) Differential gender effects of a reduced-calorie diet on systemic inflammatory and immune parameters in nonhuman primates. Journal of Periodontal Research 43(5):500-507.
- Wulansari L, Kaboosaya B, Khan M, et al. (2018) Beneficial effects of fasting regimens on periodontal tissues in experimental periodontitis mice model. Journal of International Dental and Medical Research 11:362-369.
- Park HS, Nam HS, Seo HS, Hwang SJ (2015) Change of periodontal inflammatory indicators through a 4-week weight control intervention including caloric restriction and exercise training in young Koreans: a pilot study. BMC Oral Health 15(1):109.
- Brongers HA/ FFB/ JAE/ CHJ de G/ JWR/ AS/ AS van der W. Instruction and Interpretation. Studies in Hebrew Language, Palestinian Archaeology and Biblical Exegesis. Papers Read at the Joint British-Dutch Old Testament Conference Held at Louvain, 1976. Leiden, E. J. Brill, 1977; 1977.
- Meisel P, Schwahn C, Luedemann J, John U, Kroemer HK, Kocher T (2005) Magnesium Deficiency is Associated with Periodontal Disease. J Dent Res 84(10):937-941.
- Knudsen SH, Karstoft K, Solomon TPJ (2014) Hyperglycemia abolishes meal-induced satiety by a dysregulation of ghrelin and peptide YY 3–36 in healthy overweight/obese humans. American Journal of Physiology-Endocrinology and Metabolism 306(2):E225-E231.
- Chakrabarti P, Kim JY, Singh M, et al. (2013) Insulin Inhibits Lipolysis in Adipocytes via the Evolutionarily Conserved mTORC1-Egr1-ATGL-Mediated Pathway. Mol Cell Biol 33(18):3659-3666.
- Lula EC, Ribeiro CC, Hugo FN, Alves CM, Silva AA (2014) Added sugars and periodontal disease in young adults: an analysis of NHANES III data. The American Journal of Clinical Nutrition 100(4):1182-1187.
- Jenzsch A, Eick S, Rassoul F, Purschwitz R, Jentsch H (2008) Nutritional intervention in patients with periodontal disease: clinical, immunological and microbiological variables during 12 months. Br J Nutr 101(6):879-885.
- van Woudenbergh GJ, Theofylaktopoulou D, Kuijsten A, et al. (2013) Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: the Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. The American Journal of Clinical Nutrition 98(6):1533-1542.
- Clemmons DR (2004) The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J Clin Invest 113(1):25-27.
- Allen NE, Appleby PN, Davey GK, Kaaks R, Rinaldi S, Key TJ (2002) The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol Biomarkers Prev 11(11):1441-1448.
- Kasprzak A, Kwasniewski W, Adamek A, Gozdzicka-Jozefiak A (2017) Insulin-like growth factor (IGF) axis in cancerogenesis. Mutation Research/Reviews in Mutation Research 772:78-104.
- Djiogue S, Nwabo Kamdje AH, Vecchio L, et al. (2013) Insulin resistance and cancer: the role of insulin and IGFs. Endocrine-Related Cancer 20(1):R1-R17.
- Klement RJ, Kämmerer U (2011) Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond) 8(1):75.
- Richter CK, Skulas-Ray AC, Champagne CM, Kris-Etherton PM (2015) Plant Protein and Animal Proteins: Do They Differentially Affect Cardiovascular Disease Risk? Advances in Nutrition 6(6):712-728.
- Chen GC, Zhang Z, van Dam RM, Qin LQ (2017) Nonlinear relation between animal protein intake and risk of type 2 diabetes: a dose-response meta-analysis of prospective studies. Am J Clin Nutr 105(4):1014-1016.
- Moorthi RN, Vorland CJ, Gallant KMH (2017) Diet and Diabetic Kidney Disease: Plant Versus Animal Protein. Curr Diab Rep 17(3):15.
- Staufenbiel I, Weinspach K, Förster G, Geurtsen W, Günay H (2013) Periodontal conditions in vegetarians: a clinical study. Eur J Clin Nutr 67(8):836-840.
- Zong G, Holtfreter B, Scott AE, et al. (2016) Serum vitamin B12 is inversely associated with periodontal progression and risk of tooth loss: a prospective cohort study. J Clin Periodontol 43(1):2-9.
- Osborn MO, Hornbuckle C, Stumbo P (1977) Nutritional Evaluation of Food Intake Records of Periodontal Patients. Journal of Periodontology 48(10):659-662.
- Timmerman MF, Abbas F, Loos BG, et al. (2007) Java project on periodontal diseases: the relationship between vitamin C and the severity of periodontitis. J Clin Periodontol 34(4):299-304.
- Lee HC (2001) Physiological Functions of Cyclic ADP-Ribose and NAADP as Calcium Messengers. Annu Rev Pharmacol Toxicol 41(1):317-345.
- Staudte H, Sigusch BW, Glockmann E (2005) Grapefruit consumption improves vitamin C status in periodontitis patients. Br Dent J 199(4):213-217.
- Abou Sulaiman AE, Shehadeh RMH (2010) Assessment of Total Antioxidant Capacity and the Use of Vitamin C in the Treatment of Non-Smokers With Chronic Periodontitis. Journal of Periodontology 81(11):1547-1554.
- Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B (2001) Calcium and vitamin D supplements reduce tooth loss in the elderly. The American Journal of Medicine 111(6):452-456.
- Laky M, Bertl K, Haririan H, et al. (2017) Serum levels of 25-hydroxyvitamin D are associated with periodontal disease. Clin Oral Invest 21(5):1553-1558.
- Neiva RF, Al-Shammari K, Nociti FH, Soehren S, Wang HL (2005) Effects of Vitamin-B Complex Supplementation on Periodontal Wound Healing. Journal of Periodontology 76(7):1084-1091.
- Varela-López A, Giampieri F, Bullón P, Battino M, Quiles J (2016) A Systematic Review on the Implication of Minerals in the Onset, Severity and Treatment of Periodontal Disease. Molecules 21(9):1183.
- Najeeb S, Zafar M, Khurshid Z, Zohaib S, Almas K (2016) The Role of Nutrition in Periodontal Health: An Update. Nutrients 8(9):530.
- Tanaka K, Miyake Y, Okubo H, et al. (2014) Calcium intake is associated with decreased prevalence of periodontal disease in young Japanese women. Nutr J 13(1):109.
- Kuru BE, Laleman I, Yalnızoğlu T, Kuru L, Teughels W (2017) The Influence of a Bifidobacterium animalis Probiotic on Gingival Health: A Randomized Controlled Clinical Trial. Journal of Periodontology 88(11):1115-1123.
- Schlagenhauf U, Jockel-Schneider Y. Probiotics in the Management of Gingivitis and Periodontitis. A Review. Frontiers in Dental Medicine. 2021;2.
- Slomka V, Hernandez-Sanabria E, Herrero ER, et al. Nutritional stimulation of commensal oral bacteria suppresses pathogens: the prebiotic concept. J Clin Periodontol. 2017;44(4):344-352.
- Rodriguez-Casado A (2016) The Health Potential of Fruits and Vegetables Phytochemicals: Notable Examples. Critical Reviews in Food Science and Nutrition 56(7):1097-1107.
- Jockel-Schneider Y, Goßner SK, Petersen N, et al. (2016) Stimulation of the nitrate-nitrite-NO-metabolism by repeated lettuce juice consumption decreases gingival inflammation in periodontal recall patients: a randomized, double-blinded, placebo-controlled clinical trial. J Clin Periodontol 43(7):603-608.
- Shetty P, Shetty A, Kumari S (2019) Micronutrients and Oral Health An Opportunity To Prevent Oral Diseases. Romanian Journal of Diabetes Nutrition and Metabolic Diseases 26(3):311-316.
- Perić M, Cavalier E, Toma S, Lasserre JF (2018) Serum vitamin D levels and chronic periodontitis in adult, Caucasian population-a systematic review. J Periodont Res 53(5):645-656.
- Tennert C, Reinmuth A, Bremer K, et al. (2020) An oral health optimized diet reduces the load of potential cariogenic and periodontal bacterial species in the supragingival oral plaque: A randomized controlled pilot study. Microbiology Open 9(8).


