Interspecies Transmission of Pathogen and Cascading Effect on Biodiversity Loss Sunil Kumar1*, Ravi Upadhyay2
1Saheed Bhagat Singh Government Post Graduate College, Pipariya- Narmadapuram, India
2Government Narmada Post Graduate College Narmadapuram, India
*Correspondence to: Sunil Kumar
Citation: Kumar S and Upadhyay R (2024) Interspecies transmission of pathogen and cascading effect on biodiversity loss. Sci Academique 5(1): 1-8
Received: 31 December, 2023; Accepted: 2 February, 2024; Publication: 14 February, 2024
Abstract
Biodiversity is the structural and functional unit of ecosystem. Ecosystems without biodiversity indicate poor ecological health. Biodiversity loss are triggered by the demolishing the territory, home range and niches of wildlife. The habitat loss, deforestation, fragmentation, overexploitation and introduction of alien species made them to move toward ecotone where interspecies interaction occurs frequently. Interspecies interaction results interspecies transmission of disease from wild host to domestic and ultimately to human has been reported recently. Wildlife is the major reservoir host carrying a range of pathogens. Emergence of novel pathogens on loss of biodiversity results dangerous cascading effects on human civilization that have been observed in recent decades. Apart from pathogens, there are beneficial microbiome that suppress the emergence of pathogens and protect us from infectious diseases. But, limited knowledge about microbiome and consecutive loss of biodiversity has broken the invisible defensive shield that exists around us.
Keywords: Biodiversity loss, Microbiome, Pathogens, Oval Disease model, Dilution effect
Introduction
Human mediated biological destructions are imposing an enormous stress on all components of nature. One of them is loss of biodiversity. Global population size has been declining since 1970 and population of various taxa are recently threatened with extinction 14% of birds, 26% of mammals, 40% of amphibians; 34% of gymnosperms; 33% of corals [1]. Recent studies have shown that biodiversity loss, over past decades, increased the rate of disease transmission. Emerging diseases and their related pathogens have been identified not only in humans but also among wildlife, livestocks, crops and plants. The probability of emergence of pathogens from
wildlife to humans has been positively correlated [2,3]. For example, Nipah virus translocated from wild fruit bats to domestic pigs and then spilled over from pigs to humans [4]. Similarly, Anthropogenic spread of Aedes mosquito causes emergence of Dengue and Chikunguna, Zika virus far beyond Africa [5]. The jumping of pathogens to humans is almost associated with most of the wild animals like rodents, carnivores, livestock, non-human primates and bats are to be the vector of pathogens. In other words, pathogens are limited to their wild host at their endemic places. As loss of biodiversity occurs, wild pathogens get altered genetically inducing inter-species transmissions to adopt themselves in new hosts. Thus, emerging pathogens could have been outbreaks of infectious diseases that cause long-lasting effects on human and other populations, inevitably affecting our ecosystem. COVID-19 crisis and SARS outbreak have been mounting globally in recent years. H1N1, H5N1, HIV and Nipah virus are translocated from wild animals to humans under extremely dense and physiologically stressed conditions [6,7]. Hunting and devouring of wildlife by humans profoundly changing our environment make themselves susceptible to that particular disease [8]. Nasi et al. reported that million tons of bushmeat from forest of Amazon and Congo basins are served to millions of people being interconnected with wildlife. Consumption of meat of wild animals might be cascading effects on our ecosystem and humans. The food web in an ecosystem have been disturbed on loss of many species and interfered the food chain of another’s that ultimately disturbs the energy distribution of succeeding trophic levels. Likewise, cascading extinction of many species of snake on fungal infection of amphibian’s skin is reported elsewhere. Moreover, most of the infectious diseases are controlled by microbiome that potentially interacts with pathogens and developed the concept of 3D disease model. Unfortunately, knowledge about the microbiome is either limited or unknown. In this review, Authors have discussed interspecies transmission of pathogens and cascading effects on biodiversity loss.
Interspecies Transmission of Pathogen
As human population pressure is elevated, somehow, they have encroached the habitats of wildlife resulting in the emergence of pathogens from endemic hosts to humans. Most of the wild animals are the reservoir of the pathogen existing in dormant stages within them. Humans’
interactions with wild animals make themselves susceptible to particular diseases. For example,
AIDS pandemic, Acquired Immuno Deficiency Syndrome (AIDS) of humans is caused by human immunodeficiency viruses’ types 1 and 2 (HIV-1 and HIV-2). The origins and evolution of both HIV viruses are the result of multiple cross-species transmissions of simian immunodeficiency viruses (SIVs) naturally infecting African primates. On tracing the genetic changes, it was observed SIVs crossed from monkeys to apes and subsequently to humans lead to successful host switches [9]. Highly pathogenic avian influenza A (HPAI) subtype H5N1 is repeatedly crossing the species barrier from infected poultry to humans [10,11]. Recently, in 2019, Novel COVID-19 virus derived from wild bat has been spread globally among the human population [12]. The ongoing elevation of viral population led to pandemic adaptation with human.
Infectious disease malaria is caused by Plasmodium that is transmitted by the bite of an infected female Anopheles mosquito. Loy et al. [13] reported P. falciparum evolved from a recent cross-species transmission of a parasite from a gorilla. Saprolegnia is a pathogen common in fish populations that causes embryonic mortality of amphibians in the Cascade Mountains of Oregon, U.S.A. Kiesecker et al. and Prada-Salcedo et. al. [14,15] reported multihost infection of Saprolegnia spp. in different species of fish, frogs and salamanders. The identification of Saprolegnia sp. on the body of adult specimens of dead frogs provides evidence of the ability of this fungus to be an opportunistic parasite of amphibians. Prada-Salcedo et. al. [15] concluded species of Saprolegnia may induce vertical interspecies transmission in Colombian freshwater ecosystems. Similarly, Anthrax is a potentially life-threatening bacterial disease affecting mainly wild and domesticated herbivores. But, risk of human infection is greater in those who consume contaminated animal products [16,17].
The human diarrheal disease cholera is caused by the aquatic bacterium Vibrio cholerae across the globe. V. cholerae strains specifically infect the intestinal tract of humans during consumption of infected fish or contaminated water [18]. Vibrio cholerae possesses two distinct gene pools: one is vertically inherited and globally well mixed, and the other is local and rapidly transferred across species boundaries to generate an endemic population structure [19].
Genetic aspect of pathogens emergence
Integrons, horizontal gene transfer (HGT), mutation and hybridization have played an important role in the evolution, adaptation, maintenance, and transmission of virulence. Genetic changes can alter nonpathogenic strains into new pathogenic strain if they obtain the right virulence factors. Integrons have been identified in a diverse range of bacteria that play a major role in genome evolution and help to investigate the dynamics of interspecies interactions at the genetic level [19,20]. Gene deletion, gene addition, inversion, translocation, mutation and gene decay frequently occur in lower microorganisms enabling them to proliferate in multiple hosts [21]. Thus, Interspecies transmission is a significant evolutionary event that permits the pathogens to invade new hosts. Multidisciplinary integrated knowledge of genomics, proteomics, phylogenetic relationship, and ecological condition might have facilitated comprehensive information to understand the emergence of virulent traits of pathogens on loss of biodiversity [22].
Biodiversity loss and cascading effects
Intergovernmental Science-Policy Platform for Biodiversity and Ecosystem Services (IPBES), reported loss of biodiversity by any means destabilize our ecosystem and favor the spread of pathogens. The loss of biodiversity enlarges the atrocity of disease both in wildlife as well as in the human population ultimately make us vulnerable. Biodiversity loss destroys the functioning of the ecosystem, increases the food insecurity, shortages of natural resources, health issues and economies loss and geopolitical instability. Therefore, we need to be more restrictive in regard to deforestation and wildlife food consumption [23,24]. Apart from human activities, spread of disease in wildlife causes unpredictable and precipitous loss of biodiversity.
Cascading effects of biodiversity loss on higher trophic levels are widely unclear because of lack of knowledge about the pathogens. For example, Batrachochytrium dendrobatidis (chytrid fungus) causes chytridiomycosis in which skin of amphibians are to be infected leading to the catastrophic loss of amphibians worldwide. In turn, diversity of snake community decreased on mass mortality of amphibians, a food source for snakes [25,26] an Emerging Infectious Disease (EID) is a key player in the declining amphibian populations worldwide. Emerging Infectious Diseases (EIDs) are a major threat to wildlife causes death and mass extinction of population [26] Infectious diseases caused by fungus were threatened to food security in 19th century. Late blight of potato caused by Phytophthora infestans have led to iris famine. Most of the pathogens survive in spore form in adverse conditions. But, they flourish under optimal environmental conditions and badly affect the food pyramids. Cascading effects of biodiversity loss on other taxa are largely unknown because of the baseline data are often unavailable [25]. Thus, extinction of baseline species may collapse the higher trophic level and disturb the food chain, food web and energy distribution of ecosystem.
Biodiversity and Oval Disease Model
In 3D oval disease model, there are three biologically active component exist in abiotic environment. The three biologically active components are host; microbiome and pathogen that interact with each other within environment via three ways are shown in figure 1. The parasitic interaction is to be observed between host and pathogen while, competitive interaction is to be shown between pathogen and host microbiome. Mutualism is observed between host and host microbiome. Eventually, all these components are influenced by abiotic factors of the environment. Thus, the triangular interaction of biotic component in 3D oval disease model depicts disease outcome from pathogenic zone at centre to periphery after encroaching the habitat of wild life as result of biodiversity loss. Similarly, disease pyramid determines the intensity and severity of the disease outcome from asymptomatic at the periphery to high mortality in the center of the bubble [7,27]. Therefore, the resistance and tolerance limit of hosts against pathogens are not only determined by internal immunity but are also provided by microbiomes. The microbiomes are the useful and beneficial microorganisms that protect us from the pathogen and provide vital components of immunity. Both host microbiome and environmental microbiome are interconnected with each other which act simultaneously and independently with the host. The host microbiome allows the host to adjust to its environment, similarly, environmental microbiomes establish the ecological stability.
Microbiomes regulate the abundance of endemic microbial species that can become pathogenic during colonization [3,28] have shown in an experiment that the more diverse the microbiome around the roots of wheat plants prevented the invasion of pathogenic bacterium Pseudomonas aeruginosa. In contrast, the loss of biodiversity means loss of microbiomes. The loss of microbiome exerts intensity and severity of the disease. Therefore, there are need to maintain biodiversity that sustain ecological health and facilitate the protection against pathogens.
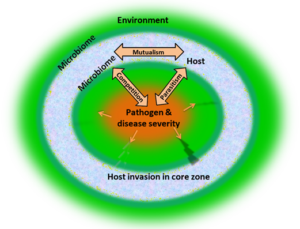
Figure 1: 3D Oval disease model: three-way interactions of i) host (mosaic) ii) host microbiome (matte green) iii) pathogen (saffron) within environment (green) depicting disease outcome from pathogenic zone at center to periphery after encroaching the habitat of wild life as result of biodiversity loss.
Dilution effect mechanism
Biodiversity may play a dual role in the emergence and transmission of infectious diseases. High biodiversity may provide a larger potential source of novel pathogens. But, well-preserved biodiversity provides immunity against outbreaks of diseases. However, recent experiments have shown that loss of biodiversity at local level increases the prevalence and transmission of infectious diseases. For example, schistosomiasis in humans is caused by Schistosoma mansoni. Host-parasite interactions have been raised on reducing diversity even if host density remains constant [3]. The dilution in the incidence of diseases can be brought about by two ways either density-dependent or frequency dependent. In ‘‘Dilution effect’’ mechanism, more species diversity in an ecosystem tends to buffer the spread of pathogens and prevents emergence of new infectious diseases. For examples, Vector-borne Lyme zoonotic disease is transmitted by ticks from white-footed mice to humans. White-footed mice in species poor region have shown greater contact with tick, whereas, species rich habitats diluted the presence of white-footed mice. In species rich habitat, ticks obtain their blood meal from these additional species which are incompetent reservoir. Blood meal diversion from the mice to alternative hosts results lower incidence of Lyme disease in humans. Thus, more diverse communities dilute the host-parasite interaction [29]. Moreover, Density-dependent disease transmission are good fit for bacterial, viral and airborne diseases in which disease outbreaks occur within-species because of the contact rate is the function of host density. However, the vector-borne diseases are likely to be frequency- dependent that increase the inter-species transmission in which contact rate being independent to population density [29]. In other word, if pathogens transmission is a function of host density then reduction in biodiversity is most likely to cause zoonotic and vector-borne disease outbreaks [30].
Conclusions
Interspecies transmission of disease and loss of biodiversity are the accelerating forces for the emergence of new pathogens. Moreover, emergence of novel pathogens are unknown to science and they are neither be detected readily nor be treated easily through vaccine, antibiotics, and quarantine. Incomplete knowledge about the countless pathogenic viruses, bacteria and fungi has enabled adverse effects not only to humans but also to the environment. Dilution mechanism of biodiversity richness tends to prevent the prevalence of existing diseases and emergence of new infectious diseases. However, defensive mechanisms of biodiversity are coupled to microbiomes that suppress the emergence of resistant strains and prevent colonization by invasive pathogenic species. Unfortunately, Humans are neglecting the cascading consequences of biodiversity loss even though knowing their importance.
References
- IUCN 2020-3 https://www.iucnredlist.org/
- Jones, K., Patel, N., Levy, M., Storeygard, A., Balk, D., Gittleman, J., & Daszak, P., 2008. Global trends in emerging infectious diseases. Nature, 451(7181), 990-993.
- Keesing, F., Belden, L., Daszak, P., Dobson, A., Harvell, C., & Holt, R. et al., 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature, 468(7324), 647-652.
- Epstein, J., Field, H., Luby, S., Pulliam, J., & Daszak, P., 2006. Nipah virus: Impact, origins, and causes of emergence. Current Infectious Disease Reports, 8(1), 59-65.
- White, R., & Razgour, O., 2020. Emerging zoonotic diseases originating in mammals: a systematic review of effects of anthropogenic land‐use change. Mammal Review, 50(4), 336-352.
- Rohr, J., Barrett, C., Civitello, D., Craft, M., Delius, B., & DeLeo, G. et al., 2019. Emerging human infectious diseases and the links to global food production. Nature Sustainability, 2(6), 445-456.
- Schmeller, D., Courchamp, F., & Killeen, G., 2020. Biodiversity loss, emerging pathogens and human health Biodiversity And Conservation, 29(11-12), 3095-3102.
- Nasi, R., Taber, A., & Van Vliet, N., 2011. Empty forests, empty stomachs? Bushmeat and livelihoods in the Congo and Amazon Basins. International Forestry Review, 13(3), 355-368.
- Sharp, P., & Hahn, B., 2011. Origins of HIV and the AIDS Pandemic. Cold Spring Harbor Perspectives In Medicine, 1(1), a006841-a006841.
- Yang, Y., Halloran, M., Sugimoto, J., & Longini, I. (2007). Detecting Human-to-Human Transmission of Avian Influenza A (H5N1). Emerging Infectious Diseases, 13(9), 1348-1353.
- Peiris, J., de Jong, M., & Guan, Y., 2007. Avian Influenza Virus (H5N1): a Threat to Human Health. Clinical Microbiology Reviews, 20(2), 243-267.
- Latinne, A., Hu, B., Olival, K., Zhu, G., Zhang, L., & Li, H. et al., 2020. Origin and cross-species transmission of bat coronaviruses in China. Nature Communications, 11(1).
- Loy, D., Liu, W., Li, Y., Learn, G., Plenderleith, L., & Sundararaman, S. et al., 2017. Out of Africa: origins and evolution of the human malaria parasites Plasmodium falciparum and Plasmodium vivax. International Journal For Parasitology, 47(2-3), 87-97.
- Kiesecker, J., Blaustein, A., & Miller, C., 2001. Transfer of a Pathogen from Fish to Amphibians. Conservation Biology, 15(4), 1064-1070.
- Prada-Salcedo, L., Franco-Correa, M., & Acosta-Galvis, A., 2011. Primer registro de Saprolegnia sp. en una población de anfibios en Colombia. Universitas Scientiarum, 16(3), 234.
- Walsh, M., Mor, S., & Hossain, S., 2019. The elephant–livestock interface modulates anthrax suitability in India. Proceedings Of The Royal Society B: Biological Sciences, 286 (1898), 20190179.
- Saile, E., & Koehler, T., 2006. Bacillus anthracis Multiplication, Persistence, and Genetic Exchange in the Rhizosphere of Grass Plants. Applied An Environmental Microbiology, 72(5), 3168-3174.
- Runft, D., Mitchell, K., Abuaita, B., Allen, J., Bajer, S., & Ginsburg, K. et al., 2013. Zebrafish as a Natural Host Model for Vibrio cholerae Colonization and Transmission. Applied And Environmental Microbiology, 80(5), 1710-1717.
- Boucher, Y., Labbate, M., Koenig, J., & Stokes, H., 2007. Integrons: mobilizable platforms that promote genetic diversity in bacteria. Trends In Microbiology, 15(7), 301-309.
- Orata, F., Kirchberger, P., Méheust, R., Barlow, E., Tarr, C., & Boucher, Y., 2015. The Dynamics of Genetic Interactions betweenVibrio metoecusandVibrio cholerae, Two Close Relatives Co-Occurring in the Environment. Genome Biology And Evolution, 7(10), 2941-2954.
- Andersson, J., & Andersson, S., 1999. Insights into the evolutionary process of genome degradation. Current Opinion In Genetics & Development, 9(6), 664-671
- Sakib, S., Reddi, G., & Almagro-Moreno, S., 2018. Environmental Role of Pathogenic Traits inVibrio cholerae. Journal Of Bacteriology, 200(15).
- Yang, N., Liu, P., Li, W., & Zhang, L., 2020. Permanentl ban wildlife 295 consumption. Science, 367(6485), 1434.2-1434.
- Chang, J., 2017. China’s Legal Response to Trafficking in Wild Animals: The Relationship between International Treaties and Chinese Law. AJIL Unbound, 111, 408-412.
- Zipkin, E., DiRenzo, G., Ray, J., Rossman, S., & Lips, K., 2020. Tropical snake diversity collapses after widespread amphibian loss. Science, 367(6479), 814-816.
- Mutnale, M., Anand, S., Eluvathingal, L., Roy, J., Reddy, G., & Vasudevan, K., 2018. Enzootic frog pathogen Batrachochytrium dendrobatidis in Asian tropics reveals high ITS haplotype diversity and low prevalence. Scientific Reports, 8(1).
- Bernardo-Cravo, A., Schmeller, D., Chatzinotas, A., Vredenburg, V., & Loyau, A., 2020. Environmental Factors and Host Microbiomes Shape Host–Pathogen Dynamics. Trends In Parasitology, 36(7), 616-633.
- Matos, A., Kerkhof, L., & Garland, J., 2005. Effects of Microbial Community Diversity on the Survival of Pseudomonas aeruginosa in the Wheat Rhizosphere. Microbial Ecology, 49(2), 257-264.
- Patil, R., Kumar, C., & Bagvandas, M., 2017. Biodiversity loss: Public health risk of disease spread and epidemics. Annals Of Tropical Medicine And Public Health, 10(6), 1432.
- Morand, S., Jittapalapong, S., Suputtamongkol, Y., Abdullah, M., & Huan, T., 2014. Infectious Diseases and Their Outbreaks in Asia-Pacific: Biodiversity and Its Regulation Loss Matter. Plos ONE, 9(2), e90032.


