Assessment of Reproductive Hormone Responses to GnRH and PGF2α Treatment Regimens in Postpartum Anoestrus Cows: A Comparative Study Theophilus Aderemi Dare1, Mohammed Mamman2, Mohammed Umaru Kawu3, Nuhu Donga Chom4, Ochuko Orakpoghenor5*
1College of Agriculture and Animal Science, Ahmadu Bello University, Mando, Kaduna, Nigeria
2Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
3Department of Veterinary Physiology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
4Radiology Unit, University Teaching Hospital, Ahmadu Bello University, Zaria, Nigeria
5Department of Veterinary Pathology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
*Correspondence to: Ochuko Orakpoghenor
Citation: Dare TA, Mamman M, Kawu MU, Chom ND, Orakpoghenor O (2024) Assessment of Reproductive Hormone Responses to GnRH and PGF2α Treatment Regimens in Postpartum Anoestrus Cows: A Comparative Study. Sci Academique 5(1): 50-60
Received: 23 March, 2024; Accepted: 3 May, 2024; Publication: 10 May, 2024
Abstract
This study aimed to compare serum profiles of follicle stimulating hormorne (FSH), luteinising hormone (LH) and progesterone (P4) in response to the administration of two doses of gonadotrophin releasing hormone (GnRH, Lecirelin®) in combination with prostaglandin F2 alpha (PGF2α, Synchromate®) ovsynch protocol in apparently healthy, post partum anestrous Friesian x Bunaji cows. Fifteen (n=15) cows were randomly divided into three groups (I, II and II) of 5 animals each. Group I (control) received 2 mL normal saline at the time of hormonal treatment; Group II received 50 µg GnRH on day 0 and day 9 and 500 µg PGF2α on day 7; Group III received 100 µg GnRH on day 0 and day 9, and 500 µg PGF2α on day 7. Blood samples were collected and assayed for FSH and LH and P4 concentrations. Peak values of FSH and LH were significantly different (p<0.05) on days 0 and 9. The plasma P4 level increased among treated cows following injection of GnRH reaching a peak on day 7 compared to the control group. It was therefore concluded that the ovsynch protocol at the dosages indicated enhanced FSH, LH and P4 profiles compared to control cows, and could initiate the onset of ovarian activity in the post partum anoestrus Friesian x Bunaji cows.
Keywords: Serum; Follicle Stimulating Hormone; Luteinizing Hormone; Plasma Progesterone; Friesian x Bunaji Cow
Introduction
The annual calf crop percentage is an indication of the reproductive efficiency of any cow-calf operation [1]. For cows to calve every year, it is imperative that the number of days such cows would be opened be regularly monitored. Anoestrus is a kind of infertility signifying a lack of oestrus expression at an expected time. It could occur after parturition as post partum or pre-service anestrus or following service as post service anoestrus when conception does not occur [2]. The appropriate manifestation of oestrus resulting from normal ovarian cyclicity is critical to successful reproductive performance in cattle [3]. A combination of genetic, physiologic, management, nutrition, cow age, bull fertility and environment has been ascribed to cause low productivity of cattle [1]. Each of these factors plays a crucial role in the reproductive efficiency of cattle. A deficit in any one area typically affects other factors, ultimately reducing the reproductive and overall performance of the herd.
Fortunately, many of the factors affecting reproductive performance can be controlled to some extent. A well planned and executed reproductive management programme coupled with the adoption of reproductive biotechnology will improve the biologic and economic efficiency of cattle breeding [1,2]. One common treatment for anoestrus or suboestrus is the ovsynch protocol [4] that does not require oestrus detection for timed artificial insemination [5]. This protocol was developed by Pursely, et al. [6] for synchronization of ovulation and consists of injection of GnRH – PGF2α – GnRH on days 0, 7, and 9, respectively. The cows are then inseminated 16-25 h after the second injection of GnRH.
The administration of GnRH produces acute increases in FSH and LH in cattle [7]. The concentrations and magnitude depend on the stage of the follicular wave and circulating steroid hormone concentrations [8]. Jensen, et al. [9] reported that the magnitude of plasma LH response increased with the increasing dosage of GnRH in diestrous heifers with the greatest increase occurring between 50 and 100 µg. However, Kohran et al. [10] showed that hormonal changes did not occur during the oestrus cycle of heifers and cattle using GnRH injection at different stages of the oestrous cycle. Therefore, the objective of the present study was to evaluate the hormonal response of post partum anoestrous Friesian x Bunaji cows to two dosage regimens of GnRH injections given at different stages of the oestrous cycle.
Material and Methods
Ethical approval: The experimental procedures were conducted in accordance with standards of the Ethics committee for animal experimentation, Ahmadu Bello University, Zaria, Nigeria via the approval No ABUCAV/2021/114.
Study site: The present study was conducted in a private farm located in Maraban Jos, Igabi Local Government Area of Kaduna State situated at a geographical coordination of 10 47′ 0″ North 7 46′ 0″ East.
Experimental animals
The cows were owned by a private farmer and maintained by semi-intensive method of production. Lactating cows from 1st to 4th parturitions weighing 200 – 250 kg and not exhibiting oestrus for 90 days or more post-partum were selected for the study. All cows received (3kg/cow) daily supplementary feeding of a mixture of cotton seed cake 48%, wheat bran 13.7%; maize 35.3%, Bone meal 2.0% and common salt 1.0%. Water and minerals licks were provided ad libitum. All cows were identified with large plastic tags and were dipped once in a week in coumaphos (Azuntol Bayer, for effective control of ectoparasites before the commencement of the study. The cows were screened for blood and helminthes parasites and appropriate treatments and vaccination against prevalent diseases were conducted. Only apparently healthy cows not suffering from any intercurrent and metabolic diseases were included.
A 5 mL blood sample was collected from the jugular vein of selected animals and placed into heparinised vacutainers. Blood samples for progesterone determination were obtained from day 1 after the first GnRH administration up to day 3 after the second GnRH administration. The blood samples in heparinised vacutainers were placed on ice immediately after collection and thereafter centrifuged at 300 g for 10 minutes within 2 hours of collection.
The plasma samples were then stored at 20 ◦C until assayed. Blood samples for FSH and LH determination were obtained just before the GnRH administrations and at 15 and 30 min, interval up to 6 h after the first and second GnRH administration. The blood samples for the determination of FSH and LH were collected into vacutainers devoid of anticoagulant and kept on ice until centrifugation at 300 g for 10 mins. The serum samples harvested were placed in separate vacutainers and stored at – 20 ◦C until assayed.
Treatment protocol
Fifteen (n=15) lactating post-partum anoestrus Friesian x Bunaji crossbred cows were selected and allotted randomly into three groups of five animals each and treated as follows.
Group 1 (n=5); control (c) All animals received 2 mL normal saline at the time of hormonal treatments.
Group II (n =5); Half dosage (HD). Animals were treated with ovsynch protocol according to Pursely et al. [6]. Accordingly, the animals received 2 mL of (50 µg) I.M injection of Lecirelin (Bioveta a.s Komen Skeho 2/2/12, Czech Republic) on day 0 followed by 2 mL (500 µg) I.M injection of PGF2α analogue Cloprostenol (Estrumate®, Schring – Plough, animal Health) on day 7 again 50 µg GnRH I.M injection on day 9.
Group III (n=5) full dosage (FD) all animals received 4 ml (100 µg) I.M injection of lecrelin (Bioveta, a.s. Komenskelo 1/1/12 Czech /Republic on day 0 and 9 and on day 7 2 mL (500 µg) I.M injection of synthetic PGF2α analogue Cloprostenol (Estrumate®, Schring Plough Animal Health).
Hormonal assays
The serum FSH and LH concentrations were determined by an immune enzymometric enzyme linked immunosorbent assay kits (Accubind Elisa, Monobind Inc. 100 North Point Drive Lake Forest, CA 92630, USA). The plasma Progesterone concentration was evaluated by a competitive progesterone ELISA kit. (Accubind Elisa, Monobind Inc). Absorbance was read at 450 nm with a micro plate reader (Spectramax ID5 Hybrid Multimode) within 20 minutes of adding stop solution. The mean absorbance value for each set of reference standards, control and samples was calculated.
Data analyses
Data generated were subjected to statistical analysis by two-way analysis of variance and fishers least significant difference LSD using Graph pad prism version 8.0 for windows graph pad software, san – Diego California USA, www.graphapd.com. Values of P<0.05) were considered statistically significant.
Results
Follicle stimulating hormone
The concentration of FSH in serum during a 360 minutes period after an injection of saline (control) or GnRH at 50µg or 100 µg are illustrated in Figure 1. No significant increase in serum FSH was observed in the control cows during a 6 h sampling period. The concentrations of serum FSH in treated cows increased above those in control groups within 15 – 30 minutes and remain elevated up to 300 minutes post GnRH administrations on day 0 and day 9. The peak concentrations of FSH in treated groups on day 9 tend to be higher than those of day 0. The serum FSH concentration returned to baseline concentrations at approximately 360 minutes post treatment on day 0 but FSH levels remain elevated in serum 360 minutes after the GnRH administration on day 9 (Figure 2).
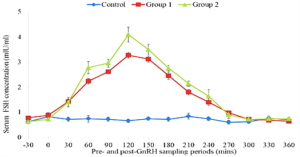
Figure 1: Mean (±SEM) serum concentrations of follicle stimulating hormone in Friesian x Bunaji cows treated with saline, 50 µg and 100 µg GnRH at day 0.
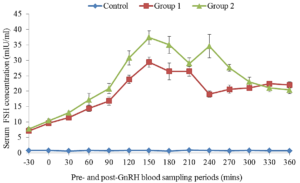
Figure 2: Mean (±SEM) serum concentrations of follicle stimulating hormone in Friesian x Bunaji cows treated with saline, 50 µg and 100 µg GnRH at day 9.
Luteinising hormone
The average serum concentration of LH during 360 minutes period after injection of saline (control) or GnRH against at 50 g or 100 g on day 0 and day 9 are illustrated in Figures 3 and 4. There were no significant changes in the serum LH of Friesian x Bunaji cows administered with saline during the 360 minutes samples period. However, the serum LH of treated groups increased above that of the control within 30 minutes and significantly by 60 to 120 minutes after GnRH treatment, and decreased to basal level by 240 minutes. The LH Concentration peaked 120 minutes after GnRH treatments and the peak concentration tend to be higher in cows treated with GnRH 48 hours after PGF2α than those treated on day 0.
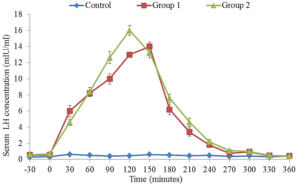
Figure 3: Mean (±SEM) serum concentrations of luteinizing hormone in Friesian x Bunaji cows (n = 5 each) administered with saline, 50 µg and 100 µg GnRH agonist at day 0.
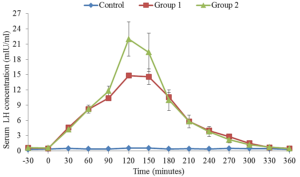
Figure 4: Mean (±SEM) serum concentrations of luteinizing hormone in Friesian x Bunaji cows (n = 5 each) administered with saline, 50 µg and 100 µg GnRH agonist at day 9.
Plasma progesterone
A highly significant variation in P4 levels among different days of the protocol (days 0, 7, and 9) was observed in treated groups with a higher value recorded on day 7. Concentrations of P4 in Plasma increased progressively after injection of GnRH and remained elevated until day 7 post injections (Figure 5). The plasma P4 concentration declined from day 8, the day after PGF2α treatment and reached their lowest around day 11. The Plasma P4 continues to rise in the control group after day 8 reaching a peak on day 12. The plasma P4 also tended to peak at higher concentration in treated groups than in controls. There was no significant difference in the pattern of P4 response to GnRH among treatment groups.
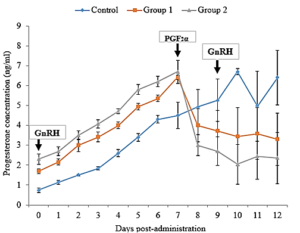
Figure 5: Mean (±SEM) plasma concentrations of Progesterone in Friesian x Bunaji cows (n = 5 each) administered with saline, 50 µg and 100 µg GnRH agonist.
Discussion
The treatment of Friesian x Bunaji cows with 50 µg and 100 µg of lecirelin, a GnRH agonist on day 0 and day 9 were able to elicit sufficient FSH an LH responses when compared to the control. This is in agreement with earlier researchers that investigated the potency of GnRH agonists in cattle [11-13]. The similar patterns in the serum LH response in treated groups are in partial agreement with Colazo et al. [7] who reported no difference in serum LH concentrations between 50 µg and 100 µg doses of GnRH in cows. However, the findings of the present study suggest a higher peak concentration with the 100 µg dosage on days 0 and 9 than the 50 µg dosage. This tends to agree with the findings of Jensen et al. [9] and partly with Colazo et al. [7] who reported that plasma concentrations of LH increased with increasing doses of GnRH. The findings of the present study also negate the findings of Kohran et al. [10] who reported that no hormonal changes occurred during the oestrus cycle of heifers and cattle following GnRH injection at different stages of the oestrous cycle. The differences in the GnRH preparations used, animal category, steroid concentrations at the time of GnRH treatment and environmental conditions have been ascribed to the inconsistency in various reports [7,14]. The serum FSH in the treated groups increased significantly above the control within 30 min of injection of GnRH, reached peak concentrations at 120 min and then returned to pre-treatment concentration at 300-360 min. This corroborates the findings of Rettmer et al. [12]. The findings of the present study also suggest that higher serum FSH release occurred on day 9 of GnRH administration than day 0. Several factors have been attributed to the magnitude of GnRH induced release of FSH. These include the stage of the follicular wave [15] and circulating steroid hormone concentrations [8,16]. Unlike day 0, the serum FSH concentration failed to return to pre-treatment concentrations by 300-360 min after GnRH administration on day 9. This could be as a result of the stage of the oestrous cycle and because the newly recruited follicles are yet to diverge into dominant and subordinate follicles when the influence of FSH is expected to decline [17].
The circulating progesterone value in groups II and III increased significantly P<0.05 on day 7 when compared to the control group. This finding corroborates the observation made by earlier workers [13,18]. The increase in Plasma P4 could be as a result of the luteo-protective and anti-luteolytic effects of the GnRH against the secretion of estradiol [12]. It could also be attributed to the formation of an accessory CL as a result of the acute increase in LH following the administration of GnRH or to the hypertrophy of the luteal cells in the spontaneous CL [19,20].
Conclusion
The treatment of anoestrus post-partum Friesian x Bunaji cows with half and full doses of GnRH agonist has a significant effect on the reproductive hormonal balance such as FSH, LH and P4 when compared to the control animals. These hormones are capable of returning anoestrus cows to cyclicity and folliculogenesis, thus improving the reproductive efficiency. The full dosage group caused higher FSH and LH release than the half dosage group. Likewise, higher FSH and LH concentrations were induced by GnRH administration on day 9 than day 0. The P4 concentrations rose significantly in treated groups over the control animals following the administration of GnRH. Further studies with more animal units are necessary to validate present findings.
Acknowledgements
The authors are grateful to the management and staff of Rahou Farms Limited, Maraban Jos, Igabi Local Government Area, Kaduna State, for the provision of experimental animals and facilities, without which the study would have been impossible.
References
- Duggan S, Pirelli G, Estil C, Ranches J, Weber DW (2021) Beef cow-calf management guide. Oregon State University Extension Service: 9327.
- Jena D, Das S, Patra BK, Biswal SS, Mohanty DN, Samal P (2006) Certain hormonal profiles of postpartum anestrous jersey cross breed cows treated with controlled internal drug release and ovsynch protocol. Veterinary World 9(10): 11 -1106.
- Degefa T, Lemma A, Jemal J, Mamo G, Tegegne A, Youngs RC (2016) Ovarian follicular dynamics in purebred and cross bred boran cross in ethiopia. African Journal of Biotechnology 15(33): 1763-1770
- Branski W, Nowicki A, Zduncyzk S (2021) Comparison of efficacy of ovsynch protocol to single PGF2α administration in treatment of individual dairy cows with post-service subestrus. Polish Journal of Veterinary Sciences 24(3): 351-354.
- Nowicki A, Baranski W, Barycyka A, Janowskin T (2017) Ovsynch protocol and its modifications in the reproductive management of dairy cattle herds – an update. Journal of Veterinary Research 61(3): 329-336.
- Pursely JR, Mee OM, Wiltbank MC (1995) Synchronisation of ovulation in dairy cows using PGF2α and GnRH. Theriogenology 44: 915-923.
- Colazo MG, Ree TO, Emmanuel DGV, Ambrose DJ (2009) Plasma luteinizing hormone concentration in cows given repeated treatments or three different doses of gonadotrophin releasing hormone. Theriogenolgy 71: 984-992.
- Nett TM, Turizillo AM, Baratta M, Rispoli LA (2002) Pituitary effects of steroid hormones on secretion of follicle stimulating hormone and luteinising hormone. Domestic Animal Endocrinology Journal 23: 33-42.
- Jensen AK, Pedersen KM, Agger JF, Madeji A (1983) Plasma luteinsing hormone response to increasing doses of synthetic gonadotrophin-releasing hormone in heifers. Acta Vet Scand 24: 211-224.
- Kohram H, Twagiramungu H, Bousquet D, Durocher J, Guilbault LA (1998) Ovarian super stimulation after follicular wave synchronization with GnRH at two different stages of the estrous cycle in cattle. Theriogenology 49: 1175-1186.
- Kaltenbach CC, Dunn TG, Kiser TE, Corah IR, Akbar AM, Niswender GD (1974) Release of FSH and LH in beef heifers by synthetic gonadotrophin releasing hormone. Journal of Animal Science 38: 357-362.
- Rettmer I, Stevenson JS, Corah LR (1992) Endocrine responses and ovarian changes in inseminated dairy heifers after an injection of a GnRH agonist 11 to 13 days after estrus Journal of Animal Science 70: 508-517.
- Picard-Hagen N, Lhermie G, Florentin S, Merie D, Frien P, Gayrad V (2015) Effect of gonadorelin, lecirelin and buserelin on LH surge, ovulation and progesterone in cattle. Theriogenology 84: 177-183.
- Hussien A, Sharawy H, Lenis YY, James D, Turna O, Risha E, et al. (2021) The impact of different estrus synchronisation programs on postpartum Holstein dairy cow reproductive performance. Mansoura Veterinary Medical Journal 21: 124-130.
- Kastelic JP, Mapletoft RJ (1998) Ovarian follicular responses in dairy cows treated with GnRH and Cloprostenol. Canadian Veterinary Journal 39:107-109.
- Karsh FJ (1987) Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinising hormone. Annual Review of Physiology 49: 365-382.
- Martinez ME, Mapletoft RJ, Kastelic JP, Carruthers T (2003) The effects of 3 gondorelin products on luteinizing hormone release, ovulation and follicular wave emergence in cattle. Canadian Veterinary Journal 44:125-131.
- Pursely JR, Martins JPN (2011) Impact of circulating concentrations of progesterone and antral age of the ovulatory follicle on fertility of high producing lactating dairy cows. Reproduction, Fertility and Development Journal 24: 267-271.
- Twagiramungu H, Guibault LA, Dufor JJ (1995) Synchronisation of ovarian follicular waves with gonadotrophin releasing hormone agonist to increase the precision of estrus in cattle: A review. Journal of Animal Science 73: 3141-3151.
- Davies TL, Mussard ML, Jimenez–Severiano H, Enright WJ, Kinder JE (2003) Chronic treatment with an agonist of gonadotrophin- releasing hormone enhances luteal function in cattle. Biology of Reproduction 69: 398-403.


