Cadmium (II) Removal by CynodonDactylon and Orange Peel Powder from Aqueous Medium by Adsorption Ashok Kr Jha*, Kiran Kumari
Department of Chemistry, Tilka Manjhi Bhagalpur University, Bhagalpur, Bihar, India
*Corresponding author: Ashok Kr Jha
Citation: Jha AK and Kumari K (2020) Cadmium (II) Removal by Cynodon Dactylon and Orange Peel Powder from Aqueous Medium by Adsorption. Sci Academique 1(1): 21-30.
Received date: 20 November, 2020; Accepted date: 07 December, 2020; Publication date: 12 December, 2020
Abstract
The research representation is based on application of Cynodondactylon and orange peel powder for the removal of Cd (II), major hazardous heavy metals of the environment. The extent of adsorption depends on pH, adsorbent dose and contact time. A comparative study of both orange peel powder and Cynodondactylon is done to know their effectiveness. An increase in percentage removal of cadmium is observed when pH is varied from 4 to 6.5. Experimental data interpret Freundlich and Langmuir isotherm describing favorable biosorption with orange peel powder. With cynodondactylon percentage removal at pH 6.5 is 46.8%. On varying pH from 6.5 to 4.00, no change in percentage removal takes place. Cynodondactylon removes cadmium through roots, stems and leaves whereas orange peel powder is effective due to its active sites. 100 ml 1 ppm Cd(II) solution is treated with 1 g orange peel powder upto different intervals of time as a result of which maximum percent removal of 65.8% is obtained. Further10 g of cynodondactylon on treatment with 100 ml 1ppm Cd solution upto 24 h, 48 h and 72 h give 0.562 ppm, 0.54 ppm and 0.532 ppm residual concentration of Cd. It may be concluded that orange peel powder is an effective adsorbent of Cd (II) from aqueous medium.
Keywords: Cynodondactylon; Orange peel; Freundlich; Langmuir; Cd (II); Biosorption
Introduction
The problem of heavy metal contamination in drinking water has attracted the attention of researchers worldwide. Arsenic and fluoride contamination in groundwater of the Gangetic plain has become very common. But case studies conducted so far have established that the Gangetic plain is now no freer of toxic heavy metal contamination [1-4]. Chromium, mercury, cobalt, cadmium and lead are some of prominent heavy metals which have been established as a potential source of health hazard [5]. Not only is this hazardous to human beings, it is also posing a threat to the aquatic kingdom. Primary sources of these heavy metal pollution may be attributed to the effluents of mining operations, tanneries, petrochemical industries, dyeing of textiles and use of heavy metals in circulatory path of computers and as well as electronics. Many of these heavy metals come into the environment and then find its path to the aquifer through leaching from the soil. Owing to non-biodegradability and persistence in soil, the heavy metals have immense possibilities of entering food chain e.g., cereals and vegetables [6]. Among these heavy metals, cadmium is also considered highly toxic element detrimental to human health. As per report in India alone 4500 industries have treatment plants for effluents and several other small industries are operating without obeying principles of green chemistry and adequate safety measures [7]. Prolonged exposure to cadmium causes renal cancer, prostate cancer, kidney disease, bone problems and emphysema. Commonly adopted methods of removal are ion exchange, treatment through limestone beds, montmoriltonite minerals, electro-dialysis and reverse osmosis. Removal by montmoriltonite minerals involvescation exchange and adsorption [8-11]. Natural and synthetic zeolites also are effective for removal of heavy metals. Industrially modified and intercalated clays also have good adsorption potential [12-14]. Adsorption through activated charcoal prepared from certain substance has also been used as an effective alternative technique but high operational cost involved has rendered it economically unfit. Natural wastes available in large quantities from agricultural practices have also the potential to be used as an alternative. Keeping in mind unused part of fruits, orange peel has been employed here as an adsorbent of Cd(II) [14]. This may not impart bad taste to the treated water unlike activated charcoal. Cynodondactylon, a perennial weed, growing in a natural way has been tried as an effective remediator of Cd(II) from aqueous medium. Removal of Cd(II) by Cynodondactylon takes place through roots, leaves and stem and the process is known as phytoremediation. Owing to abundance and low operational cost, this herb has been chosen. Other parameters such as pH, adsorbent dose and contact time have been investigated with a view to optimize the laboratory condition [15]. Particle size of orange peel powder has been fixed at 300 mesh sieve and efforts have been made to remove toxic cadmium from aqueous medium [16].
Experimental
Solution
100 ppm stock Cd(II) solution has been prepared from cadmium, nitrate
(A R grade) of Merck company. 1 ppm Cd(II) solution has been prepared from the stock solution by dilution (V1N1=V2N2).
Adsorbents
Cynodondactylon, a perennial weed, has been collected from the garden of University Department of Chemistry, Bhagalpur. It is washed with double distilled water to remove soluble impurities. After washing several times, it is soaked with filter paper and dried. 10 g Cynodondactylon is weighed and made ready for use. Orange peel powder is collected and washed from distilled water several times. After during in oven orange peel powder is powdered to 300 mesh sieves by mesh sieve available in the laboratory.
Treatment of 100 ml 1.0 ppm Cd(II) solution
When 100 ml 1.00 ppm Cd(II) solution is treated with 10 g Cynodondactylon upto 24 hours, 48 hours and 72 hours at pH 6.5. Residual concentrations on treatment with 100 ml 1.00 ppm Cd(II) solution are also measured with double beam spectrophotometer pharo 300 and the dithizone reagent supplied by Merck in Merckoquant kit. The absorbance is read at 518 nm.
Results and Discussion
|
S.NO |
Initial concentration |
Mass of orange powder |
Time |
Residual |
% Removal |
qt |
log qt |
log ct |
ct/qt |
|
1 |
1 ppm |
1 gm |
10 mins |
0.653 |
34.7 |
0.0347 |
-1.459 |
-0.185 |
18.818 |
|
2 |
1 ppm |
1 gm |
15 mins |
0.454 |
54.6 |
0.0546 |
-1.262 |
-0.342 |
8.315 |
|
3 |
1 ppm |
1 gm |
30 mins |
0.403 |
59.7 |
0.0597 |
-1.224 |
-0.394 |
6.75 |
|
4 |
1 ppm |
1 gm |
60 mins |
0.514 |
48.6 |
0.0486 |
-1.313 |
-0.289 |
10.576 |
|
5 |
1 ppm |
1 gm |
120 mins |
0.425 |
57.5 |
0.0575 |
-1.24 |
-0.371 |
7.391 |
|
6 |
1 ppm |
1 gm |
180 mins |
0.42 |
58 |
0.058 |
-1.236 |
-0.376 |
7.241 |
Table 1: Residual concentration of 100 ml Cd(II)ion after treatment with orange peel upto different intervals of time at 260c and pH6.5.
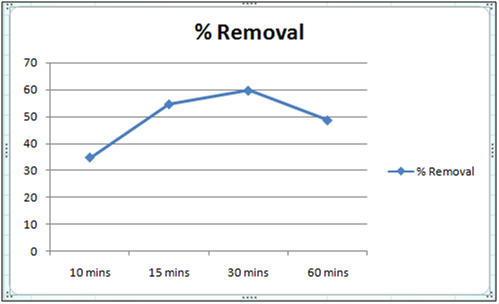
Figure 1:% removal of Cd(II)vs time with 1 gm of orange peel powder at 260c and pH 6.5.
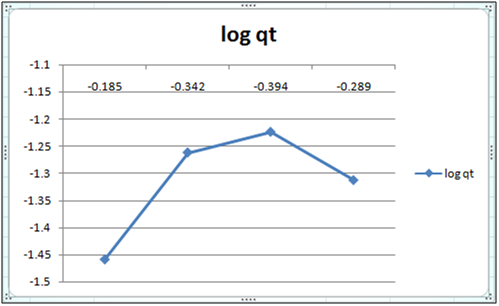
Figure 2: log qt vs log ct (Freundlich isotherm Cd(II) ion- orange peel powder system) at 260c and pH 6.5.
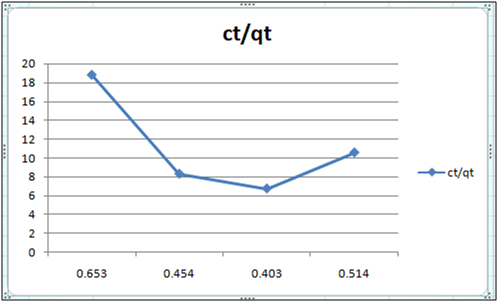
Figure 3: ct/qt vs ct (Langmuir Isotherm of Cd(II)- orange peel powder system) at 260c and pH 6.5.
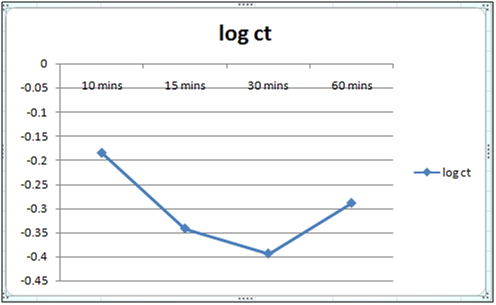
Figure 4: Plot of log of residual concentration vs time 260c and pH 6.5.
|
S.NO. |
Initial concentration |
Mass of orange powder |
Time |
Residual Concentration |
% Removal |
qt |
log qt |
log ct |
ct/qt |
|
1 |
1 ppm |
1 gm |
10 mins |
0.525 |
47.5 |
0.048 |
-1.323 |
-0.297 |
11.05 |
|
2 |
1 ppm |
1 gm |
15 mins |
0.343 |
65.7 |
0.066 |
-1.182 |
-0.464 |
5.22 |
|
3 |
1 ppm |
1 gm |
30 mins |
0.342 |
65.8 |
0.066 |
-1.181 |
-0.465 |
0.0658 |
|
4 |
1 ppm |
1 gm |
60 mins |
0.342 |
65.8 |
0.066 |
-1.181 |
-0.465 |
0.0658 |
Table 2: Residual concentration of 100 ml Cd(II)ion after treatment with orange peel upto different intervals of time at pH4.
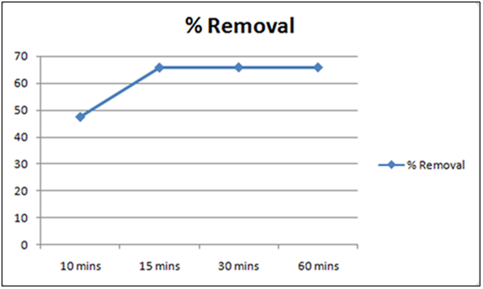
Figure 5: % removal of Cd(II)vs time with 1 gm of orange peel powder at 260c and pH 4.
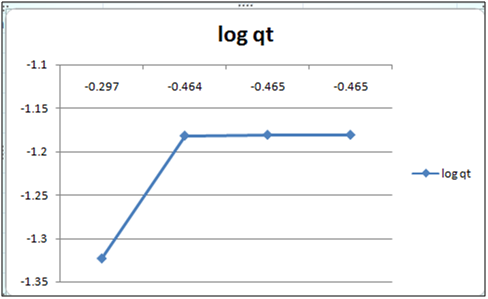
Figure 6: log qt vs log ct (Freundlich isotherm Cd(II) ion- orange peel powder system) at 260c and pH 4.
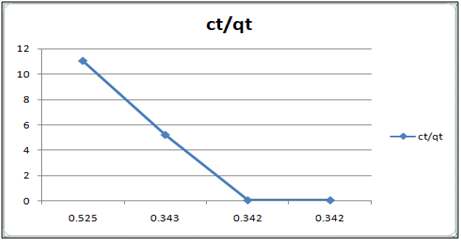
Figure 7: ct/qt vs ct (Langmuir Isotherm of Cd(II)- orange peel powder system) at 260c and pH 4.
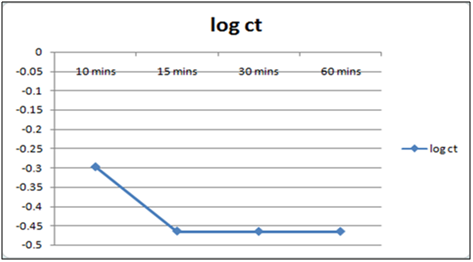
Figure 8: Plot of log of residual concentration vs. time 260c and pH 4.
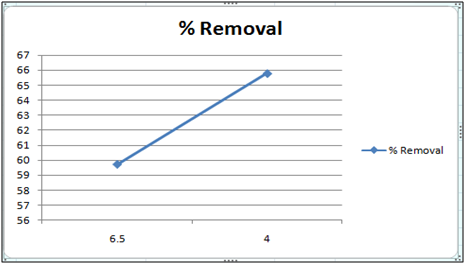
Figure 9: Effect of pH on the removal of Cd(II) by orange peel powder at 260c.
|
S.NO. |
Initial concentration |
Mass of Cyanodondactylon |
Time |
Residual Concentration |
% Removal |
qt |
log qt |
log ct |
ct/qt |
|
1 |
1 ppm |
10 gm |
24 hrs |
0.562 |
43.8 |
0.0043 |
-2.366 |
-0.25 |
130.69 |
|
2 |
1 ppm |
10 gm |
48 hrs |
0.54 |
46 |
0.0046 |
-2.337 |
-0.267 |
117.39 |
|
3 |
1 ppm |
10 gm |
72 hrs |
0.532 |
46.8 |
0.0046 |
-2.337 |
-0.274 |
115.652 |
Table 3: Residual concentration of 100 ml Cd(II)ion after treatment with Cynodondactylon upto different intervals of time at pH6.5.
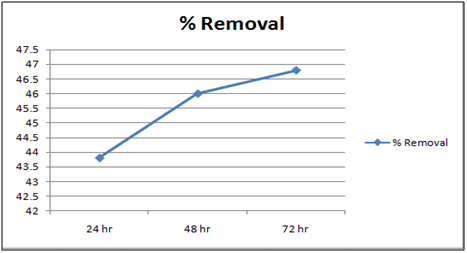
Figure 10: % removal of Cd(II)vs time with 10 gm of cynodondactylon at 260c and pH 6.5.
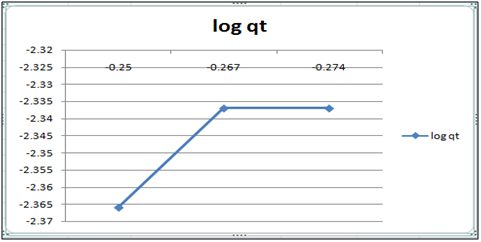
Figure 11: log qt vs. log ct (Freundlich isotherm Cd(II) ion-cynodondactylon system) at 260c and pH 6.5.
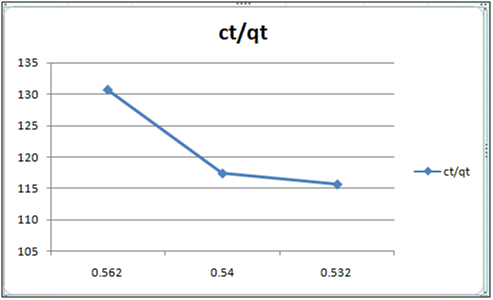
Figure 12: ct/qt vs ct (Langmuir Isotherm of Cd(II)- cynodondactylon system) at 260c and pH 6.5.
Figure 13: Plot of log of residual concentration vs. time 260c and pH 6.5.
Effect of pH
The effect of pH on the removal of Cd(II) has been studied in the pH range 4 and 6.5. The adsorbent dose has been fixed to 1 gm at 260c for orange peel powder and 10 g for Cynodondactylon. From table it is clear that maximum percentage removal is 46.8 achieved in 72 hours. Percentage removal at pH 6.5 in 24 hours, 48 hours and 72 hours are 43.8%, 46% and 46.8% respectively. Equilibrium concentration is achieved in 48 hours. There is no effect of change of pH on percentage removal in case of Cynodondactylon. Experimental results of removal by orange peel powder show that maximum percentage removal of Cd(II) is 65.8 at pH 4 which has been achieved in 30 minutes. So equilibrium agitation time is 30 minutes at pH 4. At pH 6.5 maximum percentage removals at 260c is 59.7 achieved in 30 minutes slight abnormality in residual concentration may be attributed to release or desorption after a certain time. A comparative study of removal of Cd(II) at pH 6.5 and 4 have revealed that maximum removal takes place at pH 4. This effect of pH may be explained due to increased protonation at pH 4 which provides more active sites on the surface of adsorbent [17]. The adsorbent used here is orange peel powder which contains polar functional groups along with pectin and dignin [18]. These polar functional groups may participate in chemical bonding and cation exchange capacity [19,20]. Exchange capacity of Cd(II) may be represented as:
- 2P– + Cd2+_> CdP2
- 2HP+ Cd2+->CdP2 + 2H+
Where P– and HP stands for polar sites on the orange peel powder surface.
Effect of time
Equilibrium time can be seen from the Table 1 and2both for Cynodondactylon and orange peel powder. With increasing time more surface area of orange peel powder becomes available for adsorption. Agitation time for removal of Cd(II) ion varies by orange peel powder ranging from 10 minutes to 180 minutes at pH 6.5 and 4 both. Maximum removal takes place in 30 minutes. For Cynodondactylon contact time ranges from 24 hours to 72 hours and the residual concentration in 72 hours is 0.532 ppm from an initial concentration of 1.00 ppm Cd(II) solution.
Adsorption isotherms
Adsorption isotherms have been explained by fitting the experimental data into the concerned equations [21,22]. Plot of log qt vs. log ct gives Freundlich isotherm whereas ct/qt versus ct indicates Langmuir isotherm [23]. qt is known from the formula qt=(ci-ct)/m. v
Where ci stands for initial concentration of Cd(II) solution and ct stands for concentration of Cd(II) after time t.
M stands for mass adsorbate in grams and v; volume in liters.
Figure 6 and 7 show log qt vs. log ct (Freundlich isotherm of Cd(II) ion-orange peel powder at 260c and at pH 6.5 and 4 respectively). Whereas figure 7 and 12 show ct/qt vs. ct show Langmuir isotherm. If Cd(II) ion-orange peel powder at 260c and pH 6.5 and 4 respectively. Graphs have also been plotted between log qt versus log ct and between ct/qt versus ct for Cynodondactylon represented by Figure 6 and 11. There experimental analyses and data obtained thereafter are a good fit for Freundlich and Langmuir adsorption isotherms [24,25]. Percentage removal has been put in the tables by the formula
%removal=(ci-ct)/ci*100
Where ci is the initial concentration and ct is the concentration after time t.
Conclusion Experimental analysis and data led to the conclusion that orange peel powder as an effective adsorbent of Cd (II) from aqueous medium. Cynodondactylon, a perennial herb, has also been used a phytoremediator of Cd(II). A comparative study of both orange peel powder and Cynodondactylon revealed that orange peel powder is more efficient than Cynodondactylon for remediation of Cd (II) from aqueous solutions. Further pH 4 is more suitable than pH 6 for removal of Cd(II) from aqueous medium. There is no effect of change in pH on phytoremediation of Cd(II) by Cynodondactylon. The data obtained could be helpful in designing a column for adsorption on a large scale. Thus, utilization of orange waste for removal of Cd (II) from aqueous medium has proved to be success.
References
- A.K. Jha (2019) Studies on concentration of As (III) and chromium (VI) in groundwater resources Naugachia region. Journal of Emerging Technologies and Innovative Research 6: 450-455.
- Jha AK, Gupta YC (2017) A case study of arsenic in the Koshi region of Khagaria district. Chem. Sci. Rev. Lett 6: 2120-2126.
- Jha, Kumar U (2014) A case study of arsenic and fluoride contamination in Bhagalpur district. Journal of chemical and pharmaceutical research6: 735-738.
- Jha AK, Kumar U (2016) Studies on removal of heavy metals by cymbopogon flexuous. AJAEB 10: 89-92.
- Kumar PR, Rao MV, Babu NC, Kumar PVR, Venkateswarlu P (2009) utilization of Erythrina variegate orientali’s leaf powder for the removal of cadmium, Indian Journal of chemical Technology 16: 308-316.
- Gupta VK, Jain CK, Ali I, Sharma M, Saini VK (2003) Removal of cadmium and nickel from wastewater using bagasse fly ash-a sugar industry waste. Water research, Elsevier.
- Sahu JN, Agarwal S, Meikap BC, Biswas MN (2009) performance of a modified multi-stage bubble column reactor for lead(II) and biological oxygen demand, removal from waste water using activated rice husk. Journal of hazardous materials 161: 317-324.
- Salah TA, Mohammad AM, Hassan MA (2014) Development of nano-hydroxyapatite/chitosan composite for cadmium ions removal in wastewater treatment.Journal of the Taiwan Elsevier.
- Jha AK, Mishra AK, Kumari V, Mishra B (2011) softening of hard water by bentonite minerals. Asian Journal of water, Environment and pollution 8: 93-96.
- Jha AK (2014) Adsorption of Cr(VI) and Arsenic onto Bentonite chemical science review and letters 3: 1182-1189.
- Jha AK, Majumdar S (2019) characterization of Montmorillonite minerals. Journal of Emerging Technologies and Innovative Research 6: 2019.
- Jha AK (2018) Bentonite for chemical industries. Journal of the Indian chemical society 95: 35-40.
- Jha AK (2016) Bentonite minerals and its TGA, DSC and PXRD Studies. Journal of Indian chemical society 93: 437-442.
- Nuhanoglu Y, Oguz E (2003) Removal of copper(II) from aqueous solution by biosorption on the cone biomass of Thuiu Orientallis, Process Biochem 38: 1627-1631.
- Mondal P, Majumder CB, Mohanty B (2008) Treatment of arsenic contaminated water in laboratory scale up-flow bio-column reactor. Journal of Hazardous Materials 153: 136-145.
- Biswas BK, Inoue JI, Inoue K, Ghimire KN, Harada H, et al. (2008) Adsorptive removal of As(V) and As(III) from water by a Zr(IV)-loaded orange waste gel, Journal of Hazardous Materials 154: 1066-1074.
- Monser L, Adhoum N (2009) Tartrazine modified activated carbon for the removal ofPb(II), Cd(II) and Cr(III), Journal of Hazardous Materials 161: 263-269.
- Nadeem M, Mahmood A, Shahid S, Shah S, Khalid A, et al. (2006) Sorption of lead from aqueous solution by chemically modified carbon adsorbents. J. Hazard. Mater 138: 604-613.
- Karim MR, Aijaz MO, Alharth NH, Alharbi HF (2019) Composite nanofibers membranes of poly (vinyl alcohol)/chitosan for selective lead (II) and cadmium (II) ions removal from wastewater. Ecotoxicology, Elsevier.
- Ghimire KN, Inoue K, Makino K, Miyasima T (2002) Adsorptive removal of arsenic using orange Juice residue, Sep. Sci. Technol 37: 2785-2799.
- Saxena R, Sharma S (2017) Isothermal study on removal of methyl red from aqueous solution using Aloevera leaves powder, Asian Journal of Chemistry 29: 2743-2749.
- Chatterjee R, Majumder C (2019) Modelling of adsorption process in Industrial waste water treatment- A review. J. Indian chem. soc 96: 499-506.
- Das D, Chattopadhtyay KK, Mukherjee S (2019) Magnesium incorporated graphitic carbon nitride for effective removal of fluoride ions. J. Indian chem. soc 96: 455-460.
- Li F, Shen K, Long X, Wen J, Xie X, et al. (2016) Preparation and Characterization of Biochars from Eichornia crassipes for Cadmium Removal in Aqueous Solutions. PlOS One.
- Sudha R, Srinivasan K (2015) Thermodynamic and Kinetic studies on Pb(II) removal from aqueous slolution by chemically modified citrus limettoides peel. IJEP 35: 300-311.


