Catalysts and Reactors for Synthesis Gas Production via Dry Reforming of Methane: A Review assisted with DWSIM Simulation Amol Narendra Joshi1, Sreedevi Upadhyayula2*
1Department of Chemical Engineering, Vishwakarma Institute of Technology, Bibvewadi, Pune, Maharashtra, India
2Department of Chemical Engineering, Indian Institute of Technology-Delhi, Hauz Khas, New Delhi, India
*Corresponding author: Sreedevi Upadhyayula
Citation: Joshi AN and Upadhyayula S (2021) Catalysts and Reactors for Synthesis Gas Production via Dry Reforming of Methane: A Review assisted with DWSIM Simulation. Sci Academique 2(1): 20-42.
Received date: 23 February, 2021; Accepted date: 15 March 2021; Publication date: 19 March 2021
Abstract
Dry reforming of methane (DRM) produces synthesis gas which is comprised of hydrogen (H2) and carbon monoxide (CO). The enthalpy of the reaction is 247 kJ/mol suggesting that the reaction is highly endothermic and hence enormous temperature is required for the reaction to proceed. A major drawback of this reaction is the deactivation of catalysts due to coke formation and sintering. Plenty of research work has been done to develop various catalysts – bimetallic, supported, nano-catalysts to name a few. However, there exists some lacuna in the exhaustive literature of catalysts for dry reforming of methane. Different reactors have proved to be best suited for dry reforming of methane – membrane reactor, fixed bed reactor, plasma reactor, and microreactor. So, an attempt has been made in the present review paper to compile all the data available and analyze the catalysts which reduce coke formation, sintering; analyze various advancements in the synthesis methods of these catalysts and their structure along with the reaction mechanism. The experimental analysis is simulated in DWSIM software to check for the effect of variations in pressure and mole fractions on the reactant conversion.
Keywords: Catalysts; Coke; Dry reforming; Endothermic; Methane
Introduction
Carbon dioxide and methane are major green-house gases arising from anthropogenic activities and they must be reduced to decrease global warming and climate change. CH4 is 21 times more potent than CO2 to increase atmospheric temperature [1]. Dry Reforming of Methane (DRM) has gained attention by researchers in present years due to its environmental benefit and effective utilization of CO2 and CH4 for the production of producer gas, also known as synthesis gas which mainly consists of Carbon monoxide (CO) and Hydrogen (H2). This synthesis gas can be utilized for oil production using Fischer-Tropsch synthesis, oxygenated chemicals like methanol, and other value-added products [2,3]. Dry reforming of Methane is a cheaper process to obtain synthesis gas as there is no need of separating the products [4].
The DRM reaction equation is as follows:
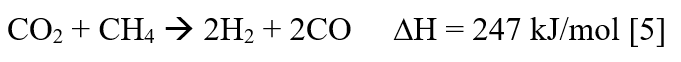
The value of ∆H is positive, suggests that the reaction is highly endothermic and hence the reaction requires a very high temperature to proceed forward. The instability of catalyst at the reaction conditions is one of the reasons for DRM not being used on an industrial scale [6]. The other reason being catalyst deactivation due to coke formation [7] and sintering. At the reaction conditions, a series of side reactions also take place, most important being the water gas shift reaction (WGS) [8]:
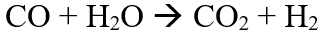
Also, Boudouard reaction is one of the side reaction which forms carbon.
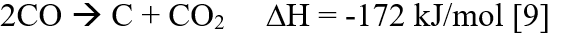
CO2 utilization could be the bridging gap for the deployment and geographical constraints of capture technologies based on boosting legislative schemes [10]. Thus, CO2 reutilization is important to curb its green-house effects.
The catalysts used for DRM are comprised of two main types of metals – noble metals and non-noble metals. The noble metals have high stability and considerably high catalytic activity. But these noble metals are expensive and availability is limited so this causes limitations in commercial use. Hence non-noble metals are widely used for DRM. Various approaches have been studied by researchers to minimize coke formation, synthesize stable catalysts, optimize reaction time and temperature to increase conversion of methane and carbon dioxide, and increase the yield of carbon monoxide and hydrogen in the ratio 1:1. Few research articles depict the use of different types of reactors like membrane reactor, fixed bed reactor, plasma reactor, microreactor, etc. for DRM. Gregor D. Wehinger, et al. performed studies on packed bed reactor using CFD packed bed being majorly used for DRM [11]. Major work has been done in synthesizing various catalysts using Ni, zeolites, minerals, actinides, bimetallic catalysts, nano-catalysts and to name a few with enhanced activities, stability, and better performance. But still, there is some sort of gap in the literature review solely devoted to different catalysts, synthesizing methods of catalysts. The conversion of CH4 and CO2 to syngas using low-cost nickel catalysts has attracted considerable interest in the clean energy and environment field. Ni single-atom catalysts exhibit excellent resistance to carbon deposition and high atom efficiency with a high reaction rate [12]. Therefore, an attempt is made to review all possible catalysts for the process of dry reforming of methane.
Comparison of different reforming processes
Reactions
CH4 can undergo various reforming processes like dry reforming, steam reforming, combination of both [13], partial oxidation of methane [14]. The reactions are given as below:
CH4 + CO2 ⇌ 2CO +2H2 ∆Ho = 247 kJ/mol….(1)
CH4 + H2O ⇌ CO +3H2 ∆Ho = 206.8 kJ/mol…..(2)
CH4 + 1/2O2 → CO + 2H2 ∆Ho=-35.6 kJ/mol….(3)
3CH4+2H2O+CO2 ⇌ 4CO+8H2 ∆Ho=660.9 kJ/mol….(4)
7CH4 + 3O2 + H2O → 7CO + 15H2 + CO2 ∆Ho = – 6.8 kJ/mol……(5)
Comparison
Steam reforming of methane as shown in reaction (2), requires a large heat supply, and the products formed are in the ratio CO:H2 = 1:3 which is not useful for Fischer Tropsch synthesis. Deactivation of the catalyst by coke formation is a major issue here. The partial oxidation of methane – reaction (3) is an exothermic process, is economical than steam reforming, can work without catalyst, and give CO:H2 = 1:2 which is then utilized for downstream Fisher Tropsch synthesis but partial oxidation requires pure O2 and high purity separation air separation plant is required and reaction equipments to handle high temperatures up to 1200K [15]. Reaction equation (5) is autothermal reforming of methane [15]. This process is slightly exothermic and is economical than steam reforming but here extensive control systems are required for proper fuel combustion which is a major challenge. Dry reforming of methane as depicted in reaction equation (1) gives CO:H2 in the ratio 1:1. Reaction equation (4) is combined dry and steam reforming, is endothermic and requires high energy to sustain the reaction.
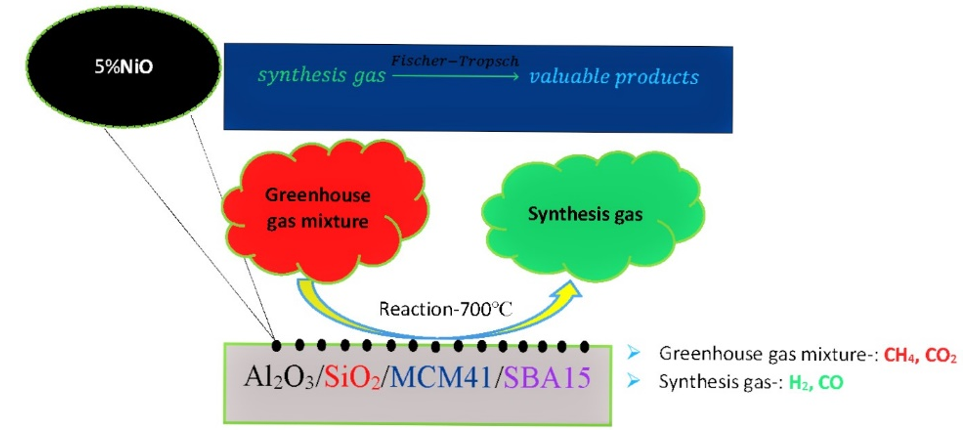
Figure 1: General Scheme of Dry Reforming of Methane [16].
Catalysts used in DRM
Metals used in catalysts are divided into two parts- base metals and noble metals. Generally, Ni is used as active species for DRM catalysts due to its high activity and low cost. Some other metals as Co or noble metals are added to examine bimetallic effects on the catalysts. Noble metals (Ru, Rh, Pd, and Pt) have high activity and low sensitivity for carbon deposition/coke formation.
The industrial choice of metal catalysts is Ni because of ease of availability, low cost but this Ni tends to get deactivated by carbon deposition. So various advancements have been to done to increase the stability of catalysts, minimize carbon deposition and develop recyclable catalysts.
Role of Promoters
Julian R.H. Ross, Bhari Mallanna Nagaraja, Dmitri A. Bulushev have developed potassium doped Ni-MgO-ZrO2 catalyst [17]. MgO, TiO2, ZrO2, La2O3 interact with Ni to significantly inhibit carbon deposition. Alkali metal like potassium (K) acts as a promoter in small amounts (0.2-0.5 % by weight). Potassium acetate and Ni in the ratio 0.125 pose strong resistance to carbon deposition.
Ye Wang Lu, et al. synthesized ZrO2 supported Ni catalyst with Si as a promoter. The catalyst was synthesized by the impregnation method with a Ni nanoparticle size of 6-9 nm. The reaction was carried out at two temperatures 400oC and 450oC. Coke deposits were observed at both temperatures but at 450oC, the coke formed could not be removed because the CO2 preferred to react with H species and not with carbon [18].
Yu. M, et.al synthesized catalysts with Sn, Ce, Mn, Co as promoters on NixMg-xO [19]. Bottom-up approach was used for synthesis. Here Sn and Co get evenly distributed whereas Mn and Ce remain segregated. Hence Sn and Co have greater resistance to coke formation and inhibit β-Carbon deposit.
Guo xia Zhang, et al. added Gd as a promoter on Ni/Al2O3-CeO2 catalyst. The precursor used was Gd(NO3)3.6H2O. One-pot Pechini method was used for catalyst synthesis [20]. Along with catalyst (200 mg), some sand particles were put to eliminate the bed temperature gradient. Weight percent of Gd was 0.8%, 1%, 1.2%, 1.4% and 2%. The reaction was carried out at 800oC. 1.2% Gd2O3 was found to be best suited to have high catalytic performance, minimum coke forming ability. Gd addition weakens the NiAl2O4 phase on the catalyst and hence enhances performance due to the reduction of Ni.
Brian Ashley developed a patented catalyst comprised of Ni on Rhenium promoter (Re) [21]. Different metal core catalysts can be alloyed with Co, Fe, Cr, etc. An electrotherm tube furnace was used for the reaction at 700oC, 750oC. In the feed stream, the ratio was CH4:CO2:Ar = 1:1:8. Re promoted catalyst performed well by 10% at 750-800oC. When the temperature was reduced to 700oC and an additional Re 0.2% was added, conversion of CH4 and CO2 doubled [21].
Al Fatesh, et al. studied the effects of promoters like Ca, Ce, and Zr on Ni/γ-Al2O3 [22]. It was found that 3%Ni/γ-Al2O3 promoted with 0.15% Ce and 0.05% Ca gave the best performance and resulted in very less coke formation. The CH4 and CO2 conversions were found to be 94.1% and 98.3% respectively at 850oC. Al Fatesh also developed catalysts with Sr as a promoter on 5wt% Ni and 5wt% Co on γ-Al2O3 [23]. DRM reaction was carried out at 700oC. The corresponding results with Sr promoter at 700oC are listed in the Table 1.
| Sr promoter (%) | CH4 conversion (%) | CO2 conversion (%) |
| 0.0 | 86.1 | 84.5 |
| 0.25 | 85.8 | 84.2 |
| 0.50 | 84.7 | 84.2 |
| 0.75 | 84.9 | 82.3 |
| 1.0 | 83.1 | 81.9 |
Table 1: CH4 and CO2 conversion at 700oC with different wt percent of Sr promoter [23]
K. Świrk, et al. also studied Y modified Ni catalysts with DLH. Co-precipitation with yttrium (III) nitrate hexahydrate resulted in increased specific surface area, smaller Ni crystallite size, enhanced reducibility, and higher distribution of weak and medium basic sites as compared to Y-free material [24]. The reaction temperature was in the range of 600-850oC. 1.5 weight percent Y was found to be the most efficient Y concentration for stability, activity, and least deactivation of the catalyst. K. Świrk studied the effect of low Y concentration on DLH. Low Y concentration led to smaller Ni crystallite, decreased Ni reducibility, and also decreased the number of basic sites. 0.4 weight% of Y results in 84% CH4 conversion and 87% CO2 conversion at 700oC [25]. The catalyst remained stable after 5 hours of reaction run.
Effect of operating variables – Temperature and Pressure
William L. Luyben designed a process plant for dry reforming of methane and considered various design variables like temperature, pressure, feed ratio, etc. to study the effect on conversion and selectivity [26]. When feed is in the ratio of 1:1 no CO2 recovery system is required and hence operating cost decreases. When pressure is increased, conversion of reactants drops so the pressure of 1 atm is most suitable for DRM. The maximum temperature allowed is 1000oC to avoid the formation of Nickel carbide on the surface of the catalyst which causes catalyst deactivation.
Rego de Vasconcelos B et al. suggested calcium hydroxyapatite Ca10(PO4)6(OH)2 as supporting material for Ni [27]. Calcium hydroxyapatite is thermally stable and decomposes above 1000oC. Hence, using this catalyst no sintering takes place. Ni size used is 10-20 nm. The pressure is 1.6 bar. Catalyst is highly active and stable for 90 hours of the test. 700oC is the most suited temperature for high reactant conversion.
Andrzej Stankiewicz, et al. synthesized Pt/C catalysts for DRM and studied the effect of catalysts at different temperatures 600oC, 700oC, and 800oC. This was a microwave-assisted DRM. With the increasing power of the microwave, the temperature increased. As the temperature increased, conversion increases, and H2/CO ratio approaches unity at 800oC. Also, with increasing temperature, the conversion of reactants increases [28].
Clarke Palmer, et.al, used molten metal alloys as a catalyst [29]. CH4:CO2 ratio was maintained as 1:1 to get Hydrogen selectivity of 97%. A bubble column reactor is used. The carbon formed in the reaction floats on the surface of the melt and can be easily separated. The metal used is Indium (In). Ar gas is purged in the bubble column. When temperature increases, CO selectivity decreases as described by Clarke Palmer.
Effect of reactant ratio and inert
Evaporation-induced self-assembly method was used by Qinhong Wei, et al. to synthesize Ce modified alumina support after which Ni was incorporated into alumina framework by citric acid auto reduction method [30]. In the reactant gas mixture, O2 was introduced which drastically increased methane conversion but decreased carbon dioxide conversion. Three catalysts were compared – Ni/Al2O3-A, Ni/Ce-Al-O-G, and Ni/Ce-Al-O-A; where, Ni/Al2O3-A this formed by the auto-reduction method, Ni/Ce-Al-O-G this is oxygen introduced and G is for general impregnation method and Ni/Ce-Al-O-A this is oxygen is introduced and auto-reduction method is used. With CH4:CO2:O2 = 1:1:0.5 conversion of CH4 was almost 100%.
Carbon deposit: Ni/Al2O3-A (18.3%) > Ni/Ce-Al-O-G (4.4%) > Ni/Ce-Al-O-A (4%)
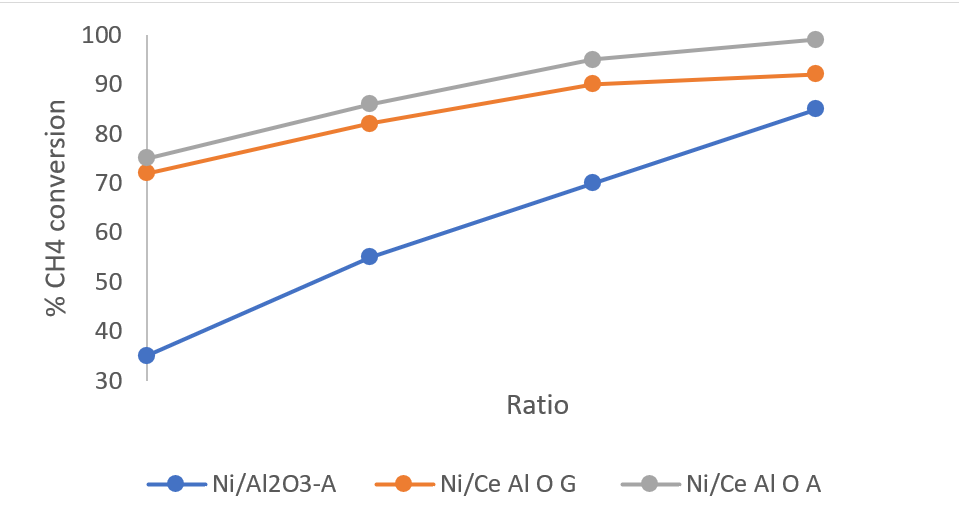
Figure 2: CH4 conversion with different ratio of CH4:CO2:O2 [30].
Ratio = 1:1:0, 1:1:0.15, 1:1:0.3, 1:1:0.5
Nano-catalysts
Takeshi Fujita, et.al developed nanoporous Ni for DRM [14]. Y2O3 has been used as a supporting material. Ni/ Y2O3 maintained high performance and suppressed carbon deposits. At 450oC, 59% conversion of methane and carbon dioxide was seen and analyzed by characterization methods.
Yasar Vafaeian, et al. used Ni/ZSM-5 catalyst with an average Ni size of 43 nm as analyzed by TEM. Transmission electron microscopy (TEM) detects the size of the ultrathin film on the surface [31]. The particle size when less than 100 nm i.e in the nano regime, the coke formation tendency decreases. Ni doping of 3-8 weight percent was used. The impregnation method was used via ultrasound irradiation. Reduction in particle size and particle size distribution is attributed to ultrasound energy during synthesis [32]. Out of which 8% Ni proved to be the best in terms of stability and resistance to coke formation.
Youngdong Song, et.al performed DRM with Ni-Mo nano-catalysts on MgO surface [33]. Mo alone is not suitable for DRM but when Ni-Mo is used improves conversion and yield. Ni:Mo was always 5:1 ratio. Helium atmosphere was given to the reactor and 50 mg catalyst loading was present. Temperature of the reactor was 800oC. Ni nanoparticles size less than 7nm were used. It was found that above 7nm carbon deposition takes place.
Meirong Lu, et.al synthesized interface-based catalyst- BN Nanoceria interface-based Ni catalyst [34]. Bao, et al.[35] suggested that syn gad formation is enhanced at the interface between metal and boron nitride (BN). So Meirong Lu, et al. confined Ni between nanoceria (NC) and boron nitride (BN). This prevented coke formation and sintering of Ni nanoparticles. OH– ions formation was fast which helped prevent coke. A tubular quartz reactor was used at 750oC. Ni/NC conversion drops after 20 hours but Ni/NC/BN conversion remains constant.
Takami, et.al suggested that plasmonic metal nanoparticles accelerate DRM [36]. Wibow, et.al showed that SrTiO3 photocatalysts can be used for photocatalytic DRM [37]. Yohei Cho, et.al developed semiconductor-supported catalysts [38]. Rhodium (Rh) and Ruthenium (Ru) nanoparticles are deposited on semiconductor support. Tantalum-based oxynitride (TaON) and nitride (Ta3N5) with Rh nanoparticles were efficient for DRM. But Ta3N5 has less thermal stability than TaON and hence Rh/TaON performed better than Rh/ Ta3N5.
Swirk K, Zhang H, et al., made NiO-Y2O3 nanostructure catalyst that was carbon resistant [39]. Yttrium loading was 3,4,8 weight percent. Double layered hydroxides (DLH) were synthesized by the co-precipitation method. When Yttrium content increases, basicity increases. Here NiY4-DLH i.e 4 weight percent Y showed the highest catalytic activity. The reaction carried out at 700oC. By Raman Spectroscopy data, Yttrium 8 weight percent gave superior coke resistance but hen conversion was not to the mark.
| Catalyst | CH4 conversion (%) | CO2 conversion (%) | H2/CO ratio |
| NiY3-DLH | 85 | 90 | 0.99 |
| NiY4-DLH | 84 | 90 | 1.00 |
| NiY8-DLH | 58 | 68 | 0.87 |
Table 2: Comparison of NiY-DLH catalysts with different Y weight% [39].
Alumina supported catalysts
N.N Gavrilova, et.al have developed a Tungsten carbide (WC) catalyst on alumina surface (Al2O3) [6]. 2.86% WC/ Al2O3 pellet 5.5 cm in length was developed. Here the temperature of the reaction can be lowered by about 100oC. The reaction rate constant is 30 times higher than the traditional catalyst used for DRM. During the reaction, water is formed as initial selectivity shows the same rate of formation for hydrogen and water. After which,
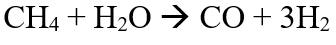
reaction is taking place. This reaction is possible only in membrane catalysts.
Ni catalyst supported on Al2O3, SiO2, and TiO2 supports, and found that the activity and selectivity were dependent on the nature of the support and increased in the order Ni/TiO2 < Ni/SiO2 < Ni/Al2O3. The low activity of the Ni/TiO2 catalyst was attributed to strong metal-support interactions. During reduction, TiO2 molecules go to the surface of Ni particles, covering active sites, and hence a decrease in the surface free energy is seen [40].
The effect of plasma using a (10% wt.) Ni/γ-Al2O3 calcined at different temperatures (300, 500, and 800°C) Results shows that plasma has indeed a positive effect on the conversion of methane and even without any catalyst the experimental setup by itself allowed a maximum conversion of methane in the range of 50% was reviewed by Jean M.L [41].
Chunshan Song and Wei Pan utilized the CO2 from electric power plants for the production of syn gas[42]. Ni catalysts were used and tri reforming of methane was performed. The process had 97% CH4 conversion and more than 80% CO2 conversion with a 1.5-2 ratio of H2:CO. The ability of catalysts to enhance the conversion of CO2 is as follows:
Yen Chen, et.al used Al promoted Ni/Palygorskite (Pal) catalyst developed by the co-precipitation method [43]. Palygorskite consists of 65.5% SiO2, 14% MgO, 5.4% Al2O3, 3.2% Fe2O3, 0.9% impurities, ignition losses are 11%. (all percent are weight percent). Ni content was 8 weight percent. Using only Ni/Pal only 5% conversion of methane was observed after 45 min. When the catalyst is Al promoted conversion enhances. Different content of Al was used – 2%, 5%, 8%, 15%. Fixed bed quartz reactor with catalyst packing was used.
Catalyst activity: Ni8/Al8/Pal > Ni8/Al5/Pal > Ni8/Al15/Pal > Ni8/Al2/Pal > Ni8/Pal [43]
Ni8/Al8/Pal and Ni8/Al15/Pal formed no carbon. Strong interaction between Ni and Al2O3 results in the formation of small Ni crystallites. Ni8/Al8/Pal is the best-suited catalyst with roughly 100% methane conversion and an H2/CO ratio of 0.98. When Al content is less than 8 weight percent, carbon deposition takes place which deactivates the catalyst [43].
Liu J-Lin, et al., conducted DRM in a Spark Plasma Reactor with Ni/Al2O3 catalyst [44]. A high AC voltage supply with a frequency of 5 kHz was given. The distance between the electrodes was 10 mm. the reactor encompasses stainless steel catalyst holders. N2 is used as an inert gas. It was observed that as methane in the feed increases coking tendency increases. Even at 50% CH4 severe coking was observed leading to plasma destabilization. 20% of the input energy is utilized in plasma for DRM endothermic reaction.
Zeolite supported catalysts
Zeolites have gained attention nowadays due to their highly porous structures. They are mainly alumino- silicates. Ningbo Gaoa, et.al studied DRM over Ni-Ce/ZSM-5 catalyst [45]. Different Ni loading was used 1%, 5%, 10%, 20%. Higher the Ni loading more was the conversion of CH4 and CO2. A fixed bed quartz reactor at 650-800oC was used.
Apanee Luengnaruemitchai, et.al studied zeolite-supported Ni catalysts, zeolite being thermally stable, has a high affinity for CO2, and enhances activity and stability [9]. 7% Ni loading gave the highest activity but also deposited carbon and deactivated the catalyst after the reaction. 91.6% conversion of CH4 was obtained. ZeoliteY proved to be best to prevent coke formation and this support has a BET area of 606.5 m2/g. A cheaper catalyst with strong potential would confine Ni and Co particles in the mesopores of SBA15 zeolite [46].
Albazari, et.al used Ni-Ce/SBA-15 [47]. The reaction temperature was 650oC giving 99% conversion of CO2 and almost 100% conversion of CH4. Ce:Ni ratio is maintained at less than 0.04 to prevent pore-blocking due to carbon deposits.
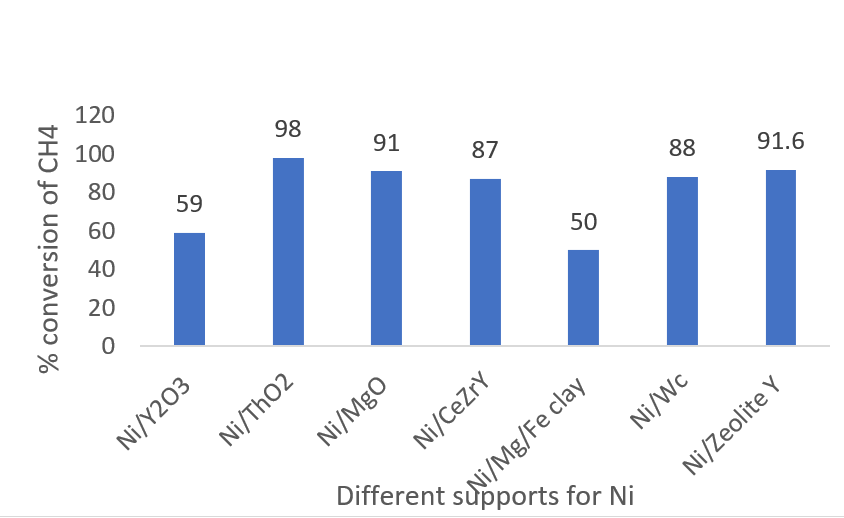
Figure 3: Conversion of CH4 over supported Ni catalysts.
Bimetallic catalysts
Ferreira AC, et.al have shown that actinide bimetallic catalyst synthesized from Uranium (U) and Thorium (Th) can stimulate the activation of CO2, prevent deactivation of the catalyst, and Uranium oxide formed helps in oxidation of carbon deposits [48]. Here 98% conversion of methane is possible and selectivity to syngas is more than 98%. Reactions are as
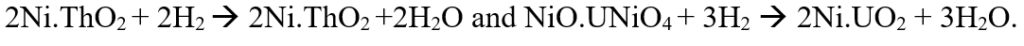
Here ThO2 after the reaction is stable but UNiO4 reduces to UO2 (U6+ to U4+). The reactions take place at 550-750oC.
Turap Y et al. synthesized Co-Ni alloy on CeO2 as a bimetallic catalyst [49]. Co here enhances oxygen adsorption due to high oxygen affinity. Ni content was 10%. Fixed bed quartz reactor at 600-850oC was used for the reaction time of 60 minutes. The atomic ratio of 0.8 of Co:Ni gave the highest conversion of methane and carbon dioxide. Comparing Ni-Co/CeO2 and Ni/CeO2, more carbon deposit was found on Ni/CeO2.
Bimetallic catalysts synthesized by the wet impregnation method have gained attention. Pan C, et al. synthesized Ni-Pd bimetallic catalyst [50]. SiO2 was used as a support material. Ni:Pd was in the ratio 4:1. The reaction temperature was 700oC and reaction time was optimized to 60 min. H2 selectivity was 98.4% and Pd was beneficial to the reduction of NiO due to the hydrogen spillover effect [51].
Ismagilov, et al. developed Ni-Co on glass fiber catalyst [52]. At 750oC temperature, the conversion of CO2 was 90% and that of CH4 was almost 100%. A small amount of carbon deposit was observed – around 0.13%.
Novel catalysts used in DRM
Very few researchers have developed catalysts with ores. Keisuke Abe, et.al used limonite laterite ore (FeOOH) as a catalyst [53]. This ore can be dehydrated to get porous Fe2O3. The reaction is 2FeOOH à Fe2O3 + H2O. This reaction proceeds at 573K. This ore also contains some Ni and 90% conversion of CO2 is achieved. After reactions, no carbon deposits on the catalyst were found.
Solar energy is considered as its solution, which requires an efficient photocatalytic DRM (PDRM). However, most catalysts explored for PDRM are not efficient, about 4% of the total solar energy. Pt/blackTiO2 catalyst with a light- diffuse- reflection- the surface of a SiO2 substrate, which created an efficient visible light PDRM at relatively low temperature [54]. But these types of catalysts have not been studied in much more detail and research is on the way.
Huang J, et al. used freeze drying to synthesize Ni catalyst [55]. Layered double hydroxides were used. Ni catalyst supported on Mg-Al oxide. A fixed bed reactor at 800oC was used for the reaction. To estimate carbon deposits, the bed was oxidized by 5% by volume O2/N2 and total CO2 and CO formed was quantified.
Katarzyna Świrk, et.al [5] developed double-layer hydroxides called hydrotalcites (HTs) which are potential carriers for metals. Then Mg2+ and Al3+ have been introduced on these carriers by co-precipitation. 5% by weight Zr is added to the catalyst to enhance stability but this limits the activity of the catalyst. Here Ni can be used which helps in decrease in reducibility of the catalyst as compared to Mg and Al. Along with Zr small amount of Y helps in increasing the activity. Also, Y improves the dispersion of Ni and decreases basicity.
Carbide catalysts have been developed and studied by Jun Guo, et al.[56]. NiMo2C catalyst was synthesized from (NH4)6Mo7O24.4H2O and Ni(NiO3)2.6H2O with Ni:Mo ratio of 2:3. A fixed bed quartz reactor at 1073K was used. No decline in NiMo2C catalytic activity was observed in 35 hours. If only Mo2C catalyst is used it deactivates due to Mo2C bulk oxidation by CO2.
Ingale P., et al. synthesized catalysts with AlOx coating on NiO/SiO2 via molecular layer deposition [57]. Alucone deposition on the catalysts initially showed lower activity due to less Ni content due to the presence of alucone by molecular layer deposition. Very few carbon deposits of 0.8% were found on spent catalysts. After the reaction, the deactivation of alucone coated catalysts was 12-18% which was much lower due to 3-6 layers of alucone.
Yanzhao Cui, et.al performed tests for partial oxidation of methane and dry reforming of methane using molybdenum phosphide catalyst in the ratio 1:1[58]. Microreactor assembly was used for reactions. It was found that molybdenum phosphide catalyst is suitable for partial oxidation of methane than dry reforming of methane. Ruckenstein, et al. used Ni on alkaline metal oxides like MgO, SrO, BaO [59]. 700oC was the reaction temperature which gave 91% and 98% conversion of methane and carbon dioxide respectively.
Sun et al. synthesized Ni–CaO–ZrO2 catalysts with different pore structures to be used as catalysts for DRM [60]. The catalyst with a mesoporous framework with a specific surface area of 210 m2/g showed both high activity and high stability. Also, no deactivation was observed after 100 hours and 700oC. The enhanced efficiency and resistance to coking were attributed to the confinement effect of the mesoporous structure which prevented Ni particles from sintering. These mesoporous materials exhibit higher activity as well as stability for DRM with retention of the mesostructure. The high specific surface area and large and uniform pore sizes inherent to these materials contributed to their superior performance. Also, the confinement effect of the mesoporous matrixes stabilized the Ni active sites during the reaction [61].
Catalyst preparation methods
Precipitation and Co-precipitation method
In precipitation method, active metal solution is precipitated by using a precipitating agent as metal carbonates and hydroxides, etc. It includes two distinct processes namely nucleation and growth. A majority of the bimetallic catalysts are synthesized by the co-precipitation method. Here, there is simultaneous precipitation of more than one component. Precipitation occurs in three steps: supersaturation, nucleation, and growth [62].
Katarzyna Świrk, et al.[5] and Yen Chen [43] have synthesized double-layered hydroxides and Ni/Palygorskite catalysts respectively by co-precipitation method. Wang S, et.al [63] synthesized Ni/MgO2 and Ni/SiO2 catalysts but these catalysts were less stable due to partial oxidation of Ni during the reaction. A base solution like NaOH is used and Na+ ions i.e alkali metal ions could act as promoters and enhance the rate [17].
Impregnation method
Many researchers have developed catalysts based on the impregnation method – wet and incipient impregnation. Impregnation method has several advantages over precipitation method (i) Filtering and washing steps are eliminated (ii) Small metal loading are easily prepared (iii) Offers some control over the metal distribution on support etc. [1]. Pan C., et al., synthesized Ni-Pd bimetallic catalysts using the wet impregnation method [49]. Ni and Pd precursors – Ni(NO3)2.6H2O and Pd(NO3)2.6H2O are used and deionized water is added. Mesoporous Silica is used as a support. Water is evaporated by sintering and powder is dried and calcinated. Oleic acid (OA) is also sometimes used and OA-assisted synthesis is performed.
Reverse Micellar Method
NiCoOx/γAl2O3 was synthesized by Abbas Beheshti, et al., using the modified reverse micellar method [64]. Ammonium oxalate, nickel acetate, and cobalt acetate are mixed in water to make 3 solutions. A mixture of cetyltriammonium bromide, hexanol, and hexane was prepared and added to the 3 solutions. Finally, all the solutions are mixed and stirred for 48 hours to get the catalyst. Centrifugation is done, washed with methanol and chloroform after which γAl2O3 is added and calcined at 400oC. This catalyst is highly stable and has high activity.
Solution Combustion Synthesis (SCS) and Paper assisted synthesis (PACS)
In this process, since we are considering Ni as the main metal for catalysts in DRM, nickel nitrate is heated in presence of glycine/urea. After which it is dried and a thick reactive gel is formed. Again when the gel is heated, combustion reaction ignites, and self-propagation through the medium takes place as synthesized by Wolf.E., et al.[65]. Ismagilov, et al. also synthesized Ni-Co catalyst on glass fiber by SCS method [49]. Danghyan V, et al., synthesized NiO-MgO catalysts by paper-assisted SCS for dry reforming of methane [66]. Catalysts formed are highly stable as small Ni crystallites easily.
Varma A., et.al synthesized NiOMgO catalysts by impregnating the reactive gel into cellulose paper [67]. The cellulose paper had a pore size of 6μm. This was then dried at 80oC and further heated at 200oC for self-ignition. 10 weight% Ni in NiOMgO prepared by PACS was most stable and active as compared to 10NiOMgO/SCS catalyst which developed 26% by weight carbon after reaction with methane conversion of only 35%.
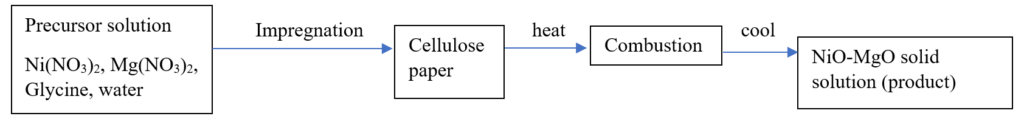
Figure 4: Cellulose paper assisted synthesis flow diagram [67].
Atomic Layer Dispersion method
Gould T.D, et.al synthesized Ni-Pt catalyst on Al2O3 support by Atomic Layer Deposition method [68]. Here the precursor is vaporized to transfer it on the support and hence a layer of the precursor is formed on the support. The pressure maintained is less than the vapour pressure. The particle size used by Gould T.D et.al was less than 3 nm which helped more and effective dispersion and also strong metal interaction.
Solvothermal process
Solvothermal synthesis is defined as a chemical reaction taking place in a solvent at temperatures above the boiling point and pressures above 1 bar. The medium used in a solvothermal synthesis can be anything water, alcohol, or any other organic or inorganic solvent [69]. Brigitte Botello Frias, et al. used a supercritical solvothermal method for the synthesis of ceria-zirconia nanostructured supports for catalysts [70]. A stainless steel reactor was used for synthesis. Two injection points one for deionized water and one for the precursor-ethanol mixture. The reactor temperature was set at 400oC and pressure was 25 MPa. Inside the reactor, the residence time was 30 seconds and after which the mixture was quenched and taken in a suspension followed by centrifugation to get nanoparticles. These samples were calcined at 800oC for 6 hours and then Ni was incorporated by wet impregnation method.
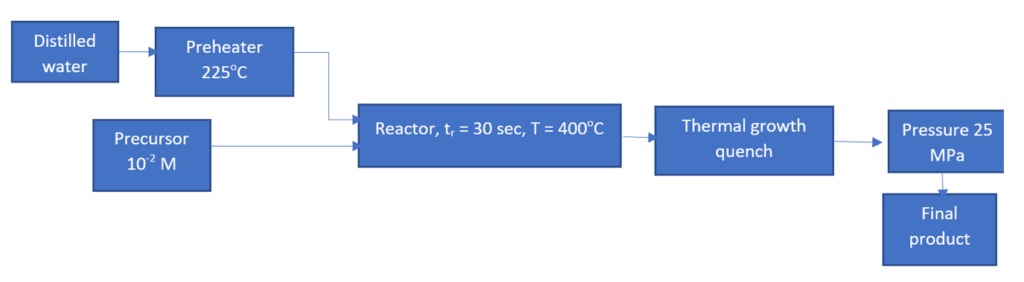
Figure 5: Solvothermal process flow diagram for Ce-Zr nano-supports [70].
Catalyst Deactivation in Dry Reforming of Methane
Deactivation
As mentioned earlier, the deactivation of catalysts in dry reforming of methane reaction is a major cause of concern. The deactivation takes place due to carbon (coke) formation on the catalysts and due to sintering of the catalysts. Sintering is nothing but the local temperature is approximately 1/3rd to ½ the catalyst melting temperature. Some stabilizers Al2O3, Magnesia, Chromia increase the melting point of catalysts [71].
Many researchers have been working to tackle this problem of deactivation mainly coke formation. Confinement of Ni between supports, metal carbide catalysts, different support materials, stabilizers has been used to curb this coke formation problem. Ce modified mesoporous silica-supported Ni catalysts provide high resistance to coke, increases the life and activity of catalyst [72]. Silica mesoporous support has a high surface area, high pore volume, large pore size which enhances contact between reactants, decreases mass transfer efficiency and Ni-based alloys on silica have high resistance to carbon deposits [73]. F. Pompeo, et al. utilized Li-SiO2 as support for Ni. Also, Ni/SiO2 was studied. It was found that on Li promoted support no carbon deposits were found whereas, on only SiO2, 6 weight percent carbon was found [74]. Many structured catalysts like monoliths, foams, and perovskite provide versatile structural properties which help in the reaction dynamics control and help in enhancing the performance and efficiency of catalysts [75].
Carbide deactivation is mainly attributed to bulk oxidation [76]. Zhiwei Yao, et al. synthesized Ni/W and Ni/Mo catalysts, and further, they were carburized to get carbide catalysts – Ni/Mo2C and Ni/WC [77]. Ni/Mo2C catalyst showed a rapid decline in the performance in 5 hours whereas Ni/WC was stable for 12 hours giving 84-88% conversion of CH4. No coke deposit was found on both the catalysts but Ni/Mo2C underwent particle sintering. The conversion of methane drops rapidly using Ni/Mo2C catalyst due to sintering.
Now a days carbon and carbon materials like carbon nanotubes (CNTs), graphene, fullerenes, etc. Wang D, et al., deposited Ni nanoparticles inside and outside CNTs by the wet chemical method [78]. Argon gas was used as inert along with the reactants. CH4:CO2:Ar = 4.5:4.5:1. Results showed that the catalytic activity of outside-CNTs was lower. Inside-CNTs were stable and gave 83.4% and 70.2% conversion of methane and carbon dioxide respectively. Characterization results showed 9.3% carbon deposits on inside-CNTs and 20.8% carbon deposits on outside-CNTs.
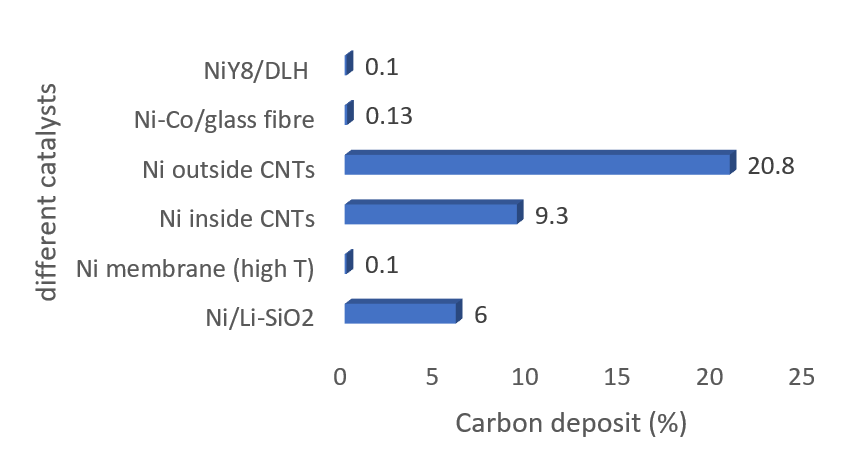
Figure 6: Carbon deposits on different catalysts.
The addition of alkaline metals to catalysts brings Lewis basic sites on the support and efficiently curbs the carbon deposition by a reverse CO disproportionation reaction [79]: CO2 + C ⇌ 2CO. Recently it was found that Ni nanoparticles with a size less than 8 nm are resistant to coke formation but are thermodynamically less stable and hence they get easily aggregated above 700oC [80]. As mentioned earlier Co has an affinity for oxygen and hence it slows down the reduction of Ni and stabilized the catalyst [81]. In a theoretical study, it was revealed Co has a low energy barrier for activation of methane and on the Co-Ni surface CH4 activates preferentially [82]. On La2O3 support many times there is poor dispersion of metals which can be improved by partial substitution of La by Ca, Ce, Sr [83] which would help in decreasing coke forming ability.
Ideally, the H2/CO ratio should be equal to 2. A higher value indicates the formation of solid carbon, a lower value indicates the presence of a reverse water-gas shift reaction. The percentage of carbon formed should be as low as possible. Ideally, the CO/CO2 ratio should be equal to 1. A higher value indicates the oxidation of the carbon deposited on the Support. The conversion of methane and carbon dioxide should be equivalent [84].
Characterization
It is important to characterize catalysts for the DRM reaction from the perspective of morphology, carbon deposition, type of carbon, phase changes, active site and support behaviours, stability, catalyst chemistry, etc. To gather this information, a wide variety of common techniques are used, including those listed below:
Reactors used for Dry Reforming of Methane
Membrane Reactor
The term membrane reactor is for those processes wherein the membrane functions as more than simply a reactive membrane – a membrane matrix used for catalyst immobilization [85]. Considering IUPAC definition, a membrane reactor is a device for simultaneously performing a reaction – steam reforming, dry reforming, autothermal reforming, etc, and a membrane-based separation in the same physical device. Hence, a membrane not only is a separator but also is a part of the reaction [86].
Jonas M. Leimert, et al. developed a membrane reactor with the capillaries made from Ni (Ni201 Material no. 2.4068) [87]. The membrane bundle consisted of 52 single capillaries with 2 mm diameter and 0.1 mm thickness. The length of the tube was 750 mm but the total heated length was 400 mm. The membrane bundle area was 0.13 m2. This reactor was proposed in replacement of Pd membranes where the operating temperature required is high. Methane conversion was found to be 70%. Membrane systems at low temperatures show carbon formation leading to membrane degradation [88]. Jonas M. Leimert, et al. found that in the regions where the temperature was 800oC, no coke formation was seen whereas in the regions where the temperature is in between 400-800oC or less coke prevailed.
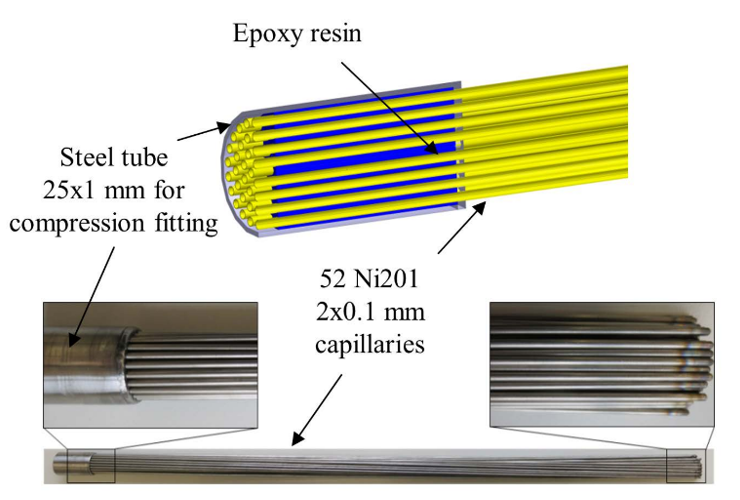
Figure 7: Membrane reactor [87].
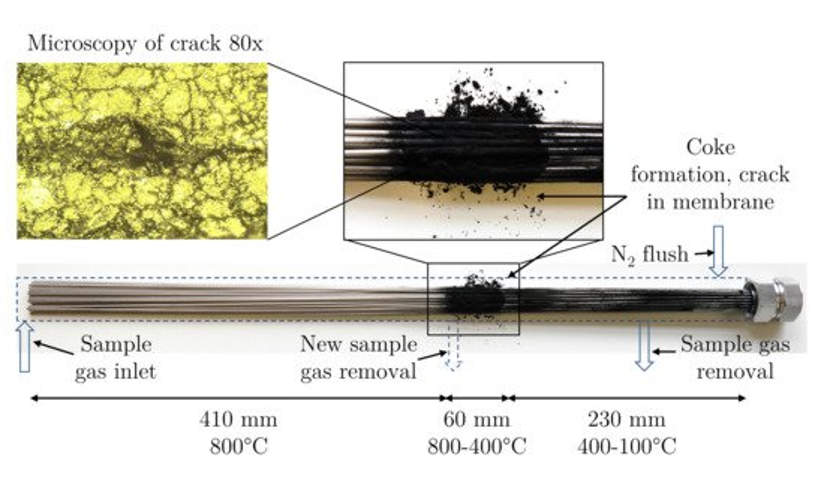
Figure 8: Coke formation in reactor (T = 400-800oC)[87]
Yang K.Z, et al. compared three different reactors – Fixed bed reactor (FBR), Straight through transport reactor (STTR), and Membrane straight through transport reactor (MSTTR) [89]. The catalyst used was Ni/Al2O3. In STTR, either an inert gas or the reactant itself transports the catalyst through the reactor. With this reactor, any possibility of catalyst decay/selectivity disguise is virtually eliminated because the catalyst and reactants are fed continuously [90]. FBR gave the lowest coke deposit (15%) but also the lowest conversion of reactants. The highest catalytic activity was found in MSTTR. 55% CH4 conversion and 95% CO2 conversion but coke formation were highest (18%). The maximum yield of H2 was seen in STTR. In MSTTR, after a certain reactor length, the hydrogen flow rate was decreased due to diffusion of H2 out of the tube with silica membrane [89]. Silica membrane being semi-permeable other species like CO, CO2, CH4, CO2 also pass out.
Fixed Bed Reactors
A fixed bed reactor (FBR), also known as a packed bed reactor consists of a bed of solid catalysts packed inside. In literature, most of the experiments are performed in a fixed bed reactor. A fixed bed reactor is economical than a membrane reactor and is easy to operate [91].
Mechanism of Dry Reforming of Methane
Considering Langmuir Hinshelwood Approach for Dry Reforming of Methane reaction with the surface reaction as the Rate Determining Step (RDS) and S1, S2 as active sites we have the following mechanism: [92]
CH4 + S1 ⇌ CH4. S1
CO2+ S2 ⇌ CO2. S2
CH4. S1 + CO2. S2 + S1⇌ 2CO + 2H2 + S2
Some researchers also suggest that CH4 is first adsorbed at an active site to yield CH2 species, which is a rate-determining step. CO2 is converted to CO by the reverse water-gas shift reaction. H2 and CO are produced via the reaction of H2O with CHx species.
The mechanism is as follows: [93]
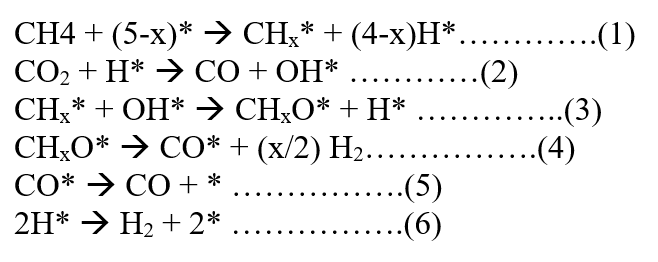
The above mechanism suggests that H* created in step (1) is essential for the adsorption and activation of CO2.
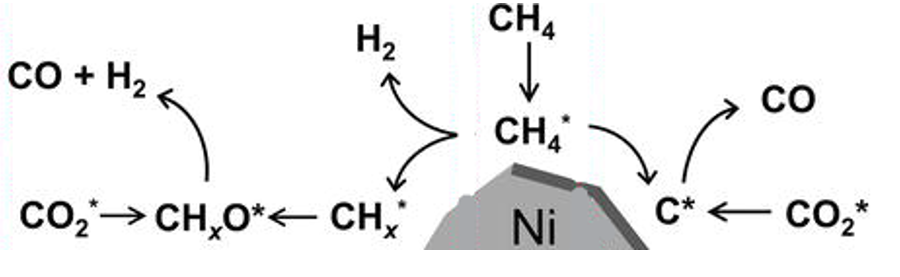
Figure 9: Reaction Mechanism scheme of DRM with supported Ni catalyst [94].
Simulation of DRM in DWSIM
DWSIM is an open software for chemical process industry simulation. Dry Reforming of Methane is simulated in DWSIM. Using a reactor, feed stream (CH4 and CO2), products (H2 and CO), and catalyst the process is simulated at 800oC and 1.01325 bar. Catalyst used is Ni (density = 8900 kg/m3). A heterogeneous catalytic reaction model is used.
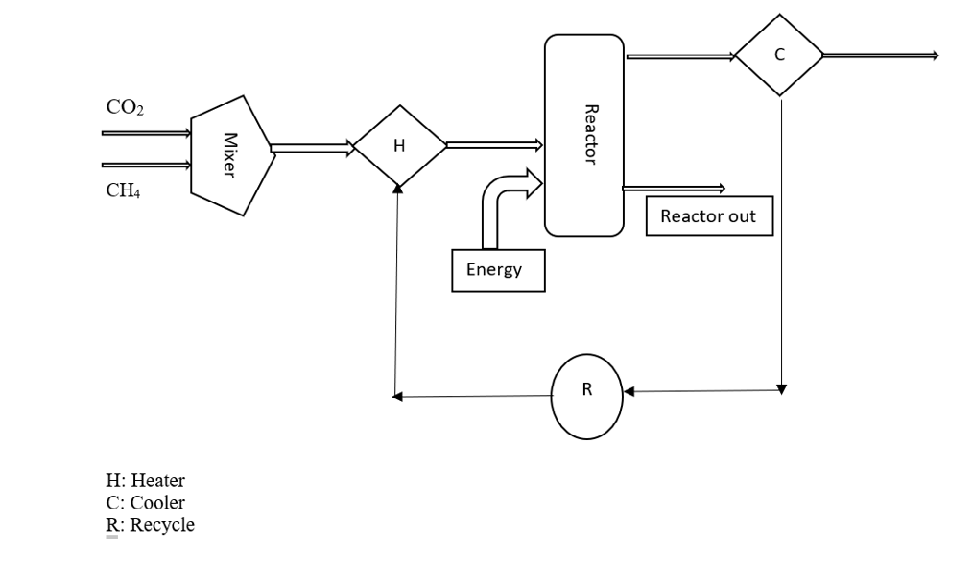
Figure 10: DWSIM Flowsheet of Dry Reforming of Methane
Using reaction kinetics of DRM, we have
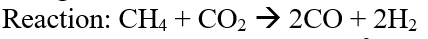
Rate= kDRM*((PCH4*PCO2) – (PH22*PCO2)/KpDRM))/((1+K1*(PCH4+K2*PCO))+(1+K3*PCO2)) [95].
Where P is the partial pressure.
From the literature, K1 = 0.50 bar-1, K2 = 9.71 bar-1, K3 = 26.21 bar-1
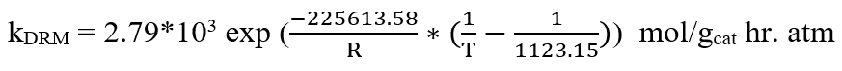
KpDRM= 147.9317, kDRM = 905.3, R = 8.314 J/mol K
Considering diameter of particle = 2 mm and Void fraction = 0.4
As particle size decreases, pressure drop increases so size is in mm.
| CH4 mole fraction | CO2 mole fraction | T (oC) | CH4 conversion (%) | CO2 conversion (%) | H2/CO ratio |
| 0.5 | 0.5 | 800 | 92.58 | 92.58 | 1.0 |
| 0.4 | 0.6 | 800 | 98.69 | 65.97 | 1.0 |
| 0.3 | 0.7 | 800 | 99.71 | 42.73 | 1.0 |
| 1.0 | 1.0 | 800 | 92.58 | 92.58 | 1.0 |
Table 4: Effect of variation of mole fraction on reactant conversion.
So, it can be seen that with the increasing mole fraction of CO2 in the feed the conversion of CH4 increases but that of CO2 decreases. Ideally, all the mole fraction ratios give H2/CO ratio of 1.0 based on the stoichiometry of the reaction.
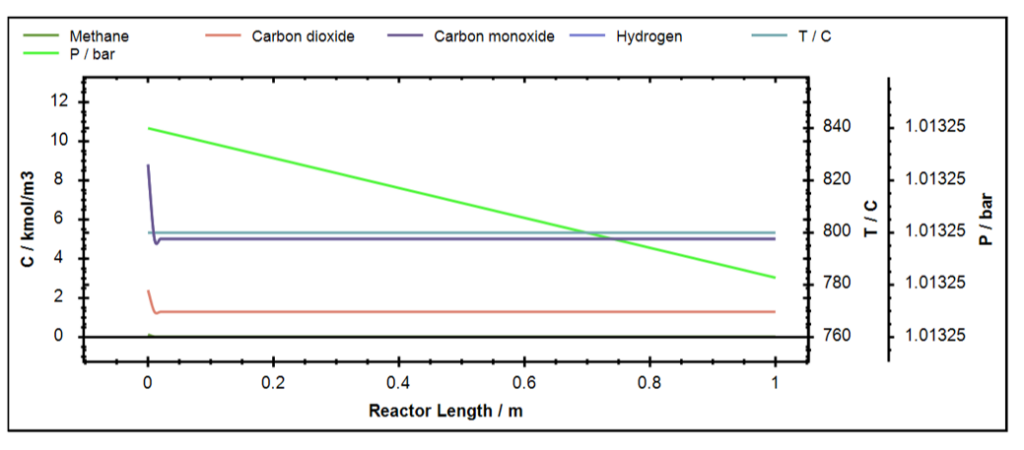
Figure 10: Concentration profile for DRM in DWSIM.
Now, when molar ratio of CH4 and CO2 is 1:1, with increase in pressure, the conversion of the reactants drops.
| Pressure (bar) | Conversion of reactants (%) |
| 1.0 | 92.67 |
| 2.0 | 86.71 |
| 3.0 | 81.83 |
| 4.0 | 77.66 |
Table 5: Effect of pressure on reactant conversion.
Conclusion
Thus, it can be inferred that Dry Reforming of Methane (DRM) is a very suitable process to convert greenhouse gases – CH4 and CO2 into valuable products like CO and H2. This process hence tries to solve the environmental issues to some extent. The endothermic nature of this process is the reason for carbonaceous deposits on the catalyst which deactivates the catalyst and hence limits the use. Nickel alone gets easily deactivated so Ni-Co catalyst is widely used for DRM. Many times, Ni on other supports like Al2O3, CeO2, MgO, SiO2, La2O3 help reducing deactivation to some extent. Various promoters like Y, Zr, Gd, alkaline earth metals are used to avoid carbon deposits. The small size of Ni (approximately < 5 nm) proves to be better performing due to better dispersion on the support. The molar ratio of CH4/CO2, the operating temperature, and pressure have considerable influence on the product molar ratio CO/H2, catalyst deactivation, equilibrium conversion of reactants, and side reactions like methane decomposition, Boudouard reaction, and water gas shift reaction. The optimum temperature for DRM is found to be in the range of 650-900oC. The pressure is 1 atm. The feed molar ratio should be CH4:CO2 = 1:1.The reaction is simulated on DWSIM software and few plots of pressure variation, mole fraction variation are plotted to verify the experimental analysis by simulation.
The above compilation of literature would try to give a pathway for new research and development in the field of dry reforming methane. The future scope of this process includes the commercialization of the process to utilize the green-house gases and the development of a catalyst that is highly stable which doesn’t get deactivated by carbon deposition and sintering to be used in process industries.
References
- Arora S, Prasad R (2016) An Overview on Dry Reforming of Methane: Strategies to Reduce Carbonaceous Deactivation of Catalysts. RSC Advances.
- Tomohiro Yabe, Sekine Y (2018) Methane conversion using carbon dioxide as oxidizing agent: A review. Fuel Processing Technology.
- Bawadi Abdullah, Ghani NAA, N.Vo DV (2017) Recent advances in dry reforming of methane over Ni-based catalysts. Journal of Cleaner Production.
- San-José-Alonso D, Juan-Juan J, Illán-Gómez MJ, Román-Martínez MC (2009) Ni, Co and Ni-Co bimetallic catalyst for dry reforming of methane. Applied Catalysis A: General.
- Świrk K, Gálvez ME, Motak M, Grzybek T, Rønning M, et al. (2018) Dry reforming of methane over Zr- and Y-modified Ni/Mg/Al double-layered hydroxides. Catcom.
- Gavrilova NN, Sapunov VN, Skudin VV (2019) Intensification of dry reforming of methane on membrane catalyst. Chemical Engineering Journal.
- Marinho ALA, Rabelo-Neto RC, Epron F, Bion N, Toniolo FS, et al. (2019) Embedded Ni nanoparticles in CeZrO2 as stable catalyst for dry reforming of methane. Applied Catalysis B: Environmental.
- Dan M, Mihet M, Lazar MD (2020) Hydrogen and/or syngas production by combined steam and dry reforming of methane on nickel catalysts. International Journal of Hydrogen Energy.
- Luengnaruemitchai A, Kaengsilalai A (2008) Activity of different zeolite-supported Ni catalysts for methane reforming with carbon dioxide. Chemical Engineering Journal.
- Koytsoumpa EI, Bergins C, Kakaras E (2018). The CO2 economy: Review of CO2 capture and reuse technologies. Journal of Supercritical Fluids 132: 3–16.
- Wehinger GD, Eppinger T, Kraume M (2015) Evaluating catalytic fixed bed reactors for Dry Reforming of Methane with Detailed CFD. Chemie Ingenieur Technik.
- Akri M, El Kasmi A, Batiot-Dupeyrat C, Qiao B (2020) Highly Active and Carbon-Resistant Nickel Single-Atom Catalysts for Methane Dry Reforming. Catalysts 10, 630.
- Gangadharan P, Kanchi KC, Lou HH (2012) Evaluation of the Economic and Environmental Impact of Combining Dry Reforming with Steam Reforming of Methane. Chem. Engg. Res. Design 90: 1956–1968.
- Takeshi Fujita, Peng X, Yamaguchi A, Cho Y, Zhang Y, et.al, (2018) Nanoporous Nickel Composite Catalyst for the Dry Reforming of Methane. ACS Omega.
- Chen WH, Lin MR, Lu JJ, Chao Y, Leu TS, et al. (2010) Thermodynamic Analysis of Hydrogen Production from Methane via Autothermal Reforming and Partial Oxidation Followed by Water Gas Shift Reaction. Int. J. Hyd. Ener.
- Fakeeha AH Kasim SO, Ibrahim AA, Abasaeed AE, Al-Fatesh AS (2019) Influence of Nature Support on Methane and CO2 Conversion in a Dry Reforming Reaction over Nickel-Supported Catalysts. Materials.
- Wang Y, Yao L, Wang Y, Wang S, Zhao Q, Mao D, et al. (2018) Low-Temperature Catalytic CO2 Dry Reforming of Methane on Ni-Si/ ZrO2 Catalyst. ACS Catalysis.
- Yu M, Zhu K, Liu Z, Xiao H, Deng W, et al. (2014) Carbon Dioxide Reforming of Methane over Promoted NixMg1-xO (111) Platelet Catalyst Derived from Solvothermal Synthesis. App. Catal. B Env: 177–190.
- Zhang G, Wang Y, Li X, Bai Y, Zheng L, et al. (2018) Effect of Gd promoter on the structure and catalytic performance of mesoporous Ni/Al2O3-CeO2 in dry reforming of methane, Kinetics, Catalysis and Reaction Engineering.
- Al-Fatesh ASA, Fakeeha AH, Abasaeed AE (2011) Effect of Selected Promoters on Ni/Al2O3 Catalyst Performance in Methane Dry Reforming. Chin J Catal 32: 1604–1609.
- Al-Fatesh A (2105) Suppression of Carbon Formation in CH4-CO2 Reforming by Addition of Sr in to Bimetallic Ni-Co/Al2O3 Catalyst. J. King. Saud. Univ.-Engg. Sci 27: 101-107.
- Świrk K, Gálvez ME, Motak M, Grzybek T, Rønning M, et al. (2018) Yttrium promoted Ni-based double-layered hydroxides for dry methane reforming, J. CO2 Util. 27: 247–258.
- Świrk K, Motak M, Grzybek T, Rønning M, Da Costa P (2018) Effect of low loading of yttrium on Ni-based layered double hydroxides in CO2 reforming of CH4, React. Kinet. Mech. Catal.
- Luyben WL (2014) Design and Control of the Dry Methane Reforming Process. Industrial and Engineering Chemistry Research.
- Rego de Vasconcelos BR, Minh DP, Martins E, Germeau A, et al. (2020) Highly-efficient hydroxyapatite-supported nickel catalysts for dry reforming of methane. International Journal of Hydrogen Energy.
- García ID, Stankiewicz A, Nigar H (2020) Syngas production via microwave-assisted dry reforming of methane, Catalysis Today.
- Palmer C, Upham DC, Smart S, Gordan MJ, Metiu H, et al. (2020) Dry reforming of methane catalysed by molten metal alloys, Nature Catalysis.
- Wei Q, Gao X, Wang L, Ma Q (2020) Rational design of nickel-based catalyst coupling with combined methane reforming to steadily produce syngas. Fuel.
- Fan MS, Abdullah AZ, Bhatia S (2009) Catalytic Technology of Carbon Dioxide of Methane to Synthesis Gas. Chem. Cat. Chem 1: 192-208.
- Vafaeian Y, Haghighi M, Aghamohammadi S (2013) Ultrasound assisted dispersion of different amount of Ni over ZSM-5 used as nanostructured catalyst for hydrogen production via CO2 reforming of methane. Energy Convers Manage 76: 1093–103.
- Song Y, Ozdemir E, Ramesh S, Adishev A, Subramanian S, et al. (2020) Dry reforming of methane by stable Ni-Mo nanocatalysts on single-crystalline MgO. Science.
- Lu M, Zhang X, Deng J, Kuboon S, Faungnawakij K, et al. (2013) Coking resistant dry reforming of methane over BN-nanoceria interface-confined catalyst. Catalysis Science and Technology RSC.
- Zhang Y, Weng X, Li H, Li H, Wei M, et al. (2015) Hexagonal Boron Nitride Cover on Pt(111): A New Route to Tune Molecule–Metal Interaction and Metal-Catalyzed Reactions. Nano Lett 15: 3616-3623.
- Takami D, Ito Y, Kawaharasaki S, Yamamoto A, Yoshida H (2019) Low temperature dry reforming of methane over plasmonic Ni photocatalysts under visible light irradiation. Sustainable Energy & Fuels.
- Wibowo S, Yamaguchi A, Shoji S, Fujita T, Abe H, et al. (2018) Photo-assisted Dry Reforming of Methane over Strontium Titanate. Chemistry Letters 47: 935-937.
- Yohei Cho, Shoji S, Yamaguchi A, Hoshina T, Fujita T, et al. (2020) Visible light driven dry reforming of methane using semiconductor supported catalyst. Chemical Communications.
- Swirk K, Zhang H, Li S, Chen Y, Rønning M, et al. (2020) Carbon-resistant NiO-Y2O3-nanostructured catalysts derived from double-layered hydroxides for dry reforming of methane, Catalysis Today.
- Bian Z, Das S, Wai MH, Hongmanorom P, Kawi S (2017) A Review on Bimetallic Nickel-Based Catalysts for CO2 Reforming of Methane. ChemPhysChem Reviews.
- Prins R (2012) Hydrogen Spillover. Facts and Fiction. Chemical Reviews.
- Chunshan S, Pan W (2004) Tri Reforming of Methane: A Novel Concept for Synthesis of Industrially Useful Synthesis Gas with Desired H2/CO Ratios Using CO2 in Flue Gas of Power Plants without CO2 Separation. Prepr. Pap.-Am. Chem. Soc., Div. Fuel Chem 49: 128-131.
- Chen Y, Chen T, Liu H, Zhang P, Wang C, et al. (2020) High catalytic performance of the Al-promoted Ni/Palygorskite catalysts for dry reforming of methane. Applied Clay Science.
- Laˇ siˇc Jurkovi´c D, Liu J-Lin, Pohar A, Likozar B (2021) Methane Dry Reforming over Ni/Al2O3 Catalyst in Spark Plasma Reactor: Linking Computational Fluid Dynamics (CFD) with Reaction Kinetic Modelling, Catalysis Today.
- Gaoa N, Cheng M, Quan C, Zheng Y (2020) Syngas production via combined dry and steam reforming of methane over Ni-Ce/ZSM-5 catalyst. Fuel.
- Aramouni NAK, Touma JG, Tarboush BA, Zeaiter J, Ahmad MN (2018) Catalyst design for dry reforming of methane: Analysis review, Renewable and Sustainable Energy Reviews.
- Albarazi A, Galvez ME, Costa PD (2015) Synthesis Strategies of Ceria–Zirconia Doped Ni/SBA-15 Catalysts for Methane Dry Reforming. Catal. Commun 59: 108112.
- Ferreira AC, Branco JB (2020) Dry reforming of methane over nanostructured nickel e Actinide (Th, U) bimetallic oxides. International Journal of Hydrogen Energy.
- Turap Y, Wang I, Fu T, Wu Y, Wang Y (2020) CoeNi alloy supported on CeO2 as a bimetallic catalyst for dry reforming of methane. International Journal of Hydrogen Energy.
- Pan C, Guo Z, Dai H, Ren R, Chu W (2020) Anti-sintering mesoporous Ni-Pd bimetallic catalysts for hydrogen production via dry reforming of methane. International Journal of Hydrogen Energy.
- Prins R (2012) Hydrogen Spillover. Facts and Fiction. Chemical Reviews.
- Abe K, Saito G, Nomura T, Akiyama T (2016) Limonitic Laterite Ore as A Catalyst for the Dry Reforming of Methane, Energy and Fuels.
- Cai X, Hu YH (2019) Advances in catalytic conversion of methane and carbon dioxide to highly valuable products. Energy Sci Eng 7: 4–29.
- Huang J, Yan Y, Saqline S, Liu W, Liu B (2020) High performance Ni Catalysts Prepared by Freeze Drying for Efficient Dry Reforming of Methane, Applied Catalysis B: Environmental.
- Jun Guo, Zhang AJ, Zhu AM, Xu Y, AU CT, et al. (2010) A Carbide Catalyst Effective for Dry Reforming of Methane at Atmospheric Pressure, Chapter 12, In Advances in CO2 Conversion and Utilization, ACS Symposium Series; American Chemical Society.
- Ingale P, Guan C, Kraehnert R, Naumann d Alnoncourt R, Thomas A, et al. (2020) Design of an active and stable catalyst for dry reforming of methane via molecular layer deposition, Catalysis Today.
- Cui Y, Liu Q, Yao Z, Dou B, Shi Y, et al. (2019) A comparative study of molybdenum phosphide catalyst for partial oxidation and dry reforming of methane, International Journal of Hydrogen Energy.
- Sun N, Wen X, Wang F, Wei W, Sun Y (2010) Effect of pore structure on Nicatalyst for CO2reforming of CH4. Energy Environ. Sci 3: 366.
- Nair MM, Kaliaguine S (2016) Structured catalysts for dry reforming of methane. New J. Chem.
- Perego C, Villa P (1997) Catalysts Preparation Methods. Catal Today. 34: 281-305.
- Wang S, Lu GQM (1998) CO2 Reforming of Methane on Ni Catalysts: Effects of the Support Phase and Preparation Technique. Appl. Catal. B Env 16: 269-277.
- Askari AB, Al Samarai M, Morana B, Tillmann L, Pfander N, et al. (2020) In Situ X-ray Microscopy Reveals Particle Dynamics in a Ni-Co Dry Methane Reforming Catalyst under Operating Conditions, ACS Catalysis.
- Wolf EE, Kumar A, Mukasyan AS (2019) Combustion synthesis: a novel method of catalyst preparation. Catalysis 31: 297-346.
- Danghyan V, Kumar A, Mukasyan A, Wolf EE (2020) An active and stable NiOMgO solid solution based catalysts prepared by paper assisted combustion synthesis for the dry reforming of methane, Applied Catalysis B: Environmental.
- Wang S, Lu GQM (1998) CO2 Reforming of Methane on Ni Catalysts: Effects of the Support Phase and Preparation Technique. Appl. Catal. B Env 16: 269-277.
- Askari AB, Al Samarai M, Morana B, Tillmann L, Pfander N, et al. (2020) In Situ X-ray Microscopy Reveals Particle Dynamics in a Ni-Co Dry Methane Reforming Catalyst under Operating Conditions, ACS Catalysis.
- Wolf EE, Kumar A, Mukasyan AS (2019) Combustion synthesis: a novel method of catalyst preparation. Catalysis 31: 297-346.
- Danghyan V, Kumar A, Mukasyan A, Wolf EE (2020) An active and stable NiOMgO solid solution based catalysts prepared by paper assisted combustion synthesis for the dry reforming of methane, Applied Catalysis B: Environmental.
- Varma A, Mukasyan A, Rogachev A, Manukyan K (2016) Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev 116: 14493-14586.
- Gould TD, Montemore MM, Lubers AM, Ellis LD, Weimer AW, et al. (2014) Enhanced Dry Reforming of Methane on Ni and Ni-Pt Catalysts Synthesized by Atomic Layer Deposition. App. Catal. A: Gen
- Nunes D, Pimentel A, Santos L, Barquinha P, Pereira L, et al. (2019) Synthesis, design, and morphology of metal oxide nanostructures, Metal oxide series.
- Frias BB, Auxéméry A, Smal E, Dziadek K, Philippot G, et al. (2020) Continuous supercritical solvothermal preparation of nanostructured ceria-zirconia as supports for dry methane reforming catalysts. The Journal of Supercritical Fluids.
- Shahid MAT (2018) Regeneration of Deactivated Ni-catalysts for CO2 Dry Reforming of Methane.
- Sangsong S, Ratana T, Tungkamani S, Sornchamni T, Phongaksorn M (2019) Effect of CeO2 loading of the Ce-Al mixed oxide on ultrahigh temperature water-gas shift performance over Ce-Al mixed oxide supported Ni catalysts. Fuel 252: 488–495.
- Li Z, Das S, Hongmanorom P, Dewangan N, Wai MH, et al. (2018) Silica-based micro- and mesoporous catalysts for dry reforming of methane. Catal. Sci. Technol 8: 2763-2778.
- Pompeo F, Nichio NN, González MG, Montes M (2005) Characterization of Ni/SiO2 and Ni/Li-SiO2 catalysts for methane dry reforming. Catal. Today. 107–108.
- Singh R, Dhir A, Mohapatra SK. et al. (2020) Dry reforming of methane using various catalysts in the process: review. Biomass Conv. Bioref 10:567–587.
- Claridge JB, York APE, Brungs AJ, Marquez-Alvarez C, Sloan J, et al. (1998) New Catalysts for the Conversion of Methane to Synthesis Gas: Molybdenum and Tungsten Carbide. J. Catalysis 180: 85-100.
- Yao Z, Jiang J, Zhao Y, Luan F, Zhu J, et al. (2016) Insights into the deactivation mechanism of metal carbide catalysts for dry reforming of methane via comparison of nickel-modified molybdenum and tungsten carbides. RSC Adv 6: 19944.
- Ma QX, Wang D, Wu MB, Zhao TS, Yoneyama Y, Tsubaki N (2013) Effect of catalytic site position: nickel nanocatalyst selectively loaded inside or outside carbon nanotubes for methane dry reforming. Fuel 108: 430–8.
- Jose-Alonso DS, Lllan-Gomez MJ, Roman-Martinez MC (2011) K and Sr promoted Co alumina supported catalysts for the CO2 reforming of methane. Catal Today 176: 187–90.
- Seo HO (2018) Recent Scientific Progress on Developing Supported Ni catalysts for Dry (CO2) Reforming of Methane. Catalysts MDPI.
- Wang H, Miller JT, Shakouri M, Xi C, Wu T, et al. (2013) XANES and EXAFS studies on metal nanoparticle growth and bimetallic interaction of Ni-based catalysts for CO2 reforming of CH4. Catalysis Today 207: 3–12.
- German ED, Sheintuch M (2013) Predicting CH4 Dissociation Kinetics on Metals: Trends, Sticking Coefficients, H Tunneling, and Kinetic Isotope Effect. J. Phys. Chem 117: 22811–22826.
- Li X, Li D, Tian H, Zeng L, Zhao ZJ, et al. (2017) Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B Environ 202: 683–694.
- Lofberg A, et al. (2017) Method of dry reforming of at least one alkane, US patent (2017), US 2017/0050845 A1.
- Matson SL, Quinn JA (1992) Membrane Reactors. In: Ho W.S.W., Sirkar K.K. (eds) Membrane Handbook. Springer, Boston, MA.
- Gallucci F, Basile A, Hai FI (2011) Introduction – A review of membrane reactors. In A. Basile & F. Gallucci (Eds.), Membranes for membrane reactors: preparation, optimization and selection (pp. 1-61). United Kingdom: John Wiley & sons.
- Leimert JM, Karl J, Dillig M (2017) Dry Reforming of Methane using Nickel Membrane Reactor, Membrane Materials, Performance and Processes.
- Basile A, Julianelli A, Longo T, Liguori S, De Falco M (2011) Pd-based Selective Membrane State-of-the-Art. In Membrane Reactors for Hydrogen Production Processes; De Falco, M.; Marrelli, L.; Iaquaniello, G., Eds.; Springer London, UK 20–56.
- Yang KZ, Twaiq F (2017) Modelling of the dry reforming of methane in different reactors: a comparative study. Reac Kinet Mech Cat 122:853–868.
- Chapter 5, Laboratory Reactors, Collection and Analysis of rate data, University of Michigan.
- Gallucci F (2012) Packed Bed Membrane Reactor. In: Drioli E., Giorno L. (eds) Encyclopedia of Membranes. Springer, Berlin, Heidelberg.
- Jun HJ, Park MJ, Baek SC, Bae JW, Ha KS, et al. (2011) Kinetics modeling for the mixed reforming of methane over Ni-CeO2/MgAl2O4 catalyst. Journal of Natural Gas Chemistry.
- Gao J, Hou Z, Lou H, Zheng X (2011) Dry (CO2) Reforming, Book Chapter 7- Fuel Cells: Technologies for Fuel Processing
- Sheng Z, Kameshima S, Sakata K, Nozaki T (November 5th 2018). Plasma-Enabled Dry Methane Reforming, Plasma Chemistry and Gas Conversion, Nikolay Britun and Tiago Silva. Intech Open


